Manuscript accepted on : August 12, 2010
Published online on: 28-12-2010
Free Radical Scavenging and Antioxidant Activity of Methanol Extract of Syzygium Aromaticum
CH. Mina Kumari*, I. Bhaskar Reddy and K. Vijaya Rachel
Department of Biochemistry, GIS, GITAM University, India.
Corresponding Author E-mail: mina.kumari80@gmail.com.
ABSTRACT: Free radical damages have been implicated for several diseases including diabetes, arthritis, cancer and ageing etc. Current research is directed towards naturally occurring antioxidants of plant origin. Methanol extract of Syzigium aromaticum (MSA) was investigated for free radical scavenging and antioxidant activity. MSA exhibited significant radical scavenging activities with IC50 values of 95 ,101, 250 , 225, 78 and 215 μg/ml against 2,2’ -diphenyl-1- picrylhydrazyl, superoxide, hydroxyl, hydrogen peroxide,metal chelating and nitric oxide respectively. Inhibition of lipid peroxidation by MSA was found to be 88 % at 1 mg/ml concentration. The result of the present study indicates that MSA can be a potential source of antioxidants.
KEYWORDS: Free radicals; antioxidant; Syzygium aromaticum; nitric oxide radical; superoxide radical
Download this article as:| Copy the following to cite this article: Kumari C. M, Reddy I. B, Rachel K. V. Free Radical Scavenging and Antioxidant Activity of Methanol Extract of Syzygium Aromaticum. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Kumari C. M, Reddy I. B, Rachel K. V. Free Radical Scavenging and Antioxidant Activity of Methanol Extract of Syzygium Aromaticum. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9377 |
Introduction
The term antioxidant refers to the activity of numerous vitamins, minerals and other phytochemicals to protect against the damage caused by reactive oxygen species (Khlifi etal.,2006). Free radicals are implicated in several degenerative diseases such as atherosclerosis, diabetes, arthritis and cancer (Halliwell, 1997). The disturbances in redox homeostasis occurring when antioxidant defenses are inadequate can damage lipids, proteins, carbohydrates and DNA.Synthetic BHA (Butylated Hydroxy Toluene) and BHA (Butylated Hydroxyl Anisole)) are carcinogenic in nature. On the basis of above facts, natural antioxidants would be promising alternatives for synthetic antioxidants (Brown et al., 1998; Liu et al.,1997).The development of alternative natural antioxidants such as those found in plants origin is of worthy consideration for our health industry and hold promising commercial potential. Medicinal plants possess a variety of compounds of known therapeutic property (Chopra et al., 1992; Ahmad et al., 1995; Harborne et al., 1995). Antioxidants have been reported to prevent oxidative damage by free radical and ROS during the occurrence of diseases like cancer and ageing. It can interfere with the oxidation process by reacting with free radicals, chelating catalytic metals and also by acting as oxygen scavenger.
Syzigium aromaticum is a tropical plant growing in South India that belongs to family of Myrtaceae. It is commonly known as clove. The unopened floral buds of cloves are reported for various medicinal properties. Aqueous extracts of clove buds are used for the treatment of dyspepsia and gastric irritation (Abasta ,1986). Phytochemical study revealed that the methanol extracts of Syzigium aromaticum contained high amount of eugenol and eugenol acetate (Kwang et al., 2001). Phytochemicals such as alkaloids, phenolics and terpins are reported to possess antioxidant activity (Steinmetz et al., 1996) .Since clove buds are used in the treatment of many diseases in traditional folk medicine and possess high amount of essential oils and flavanoids. The present investigation is aimed to carry out the free radical scavenging and antioxidant activity of methanol extract of Syzigium aromaticum.
Materials and Methods
All the chemicals used in the present study are of analytical grade and obtained from local suppliers.
Plant material
cloves of good quality were procured from spices trading corporation, Vizianagaram, Andhra Pradesh, India and authenticated by the Department of Botany, Andhra University.
Preparation of Extract
Cloves are collected and air dried for one day and is then ground to powder using an electric mill. 20 Gms of powdered cloves are extracted with 250 ml of methanol using soxhlet extractor. The extract is filtered using Whatmann (No.1) filter paper and then concentrated in vacuum to dryness. Different concentrations of methanol extracts of S.aromaticum are prepared by dissolving 10, 50, 100, 250 ,500,700 and 1000µgm of dry residue in one ml of methanol which is used for further studies. BHT (Butylated hydroxy toluene) and Quercetin are used as positive control for radical scavenging assay.
Total antioxidant activity
The total antioxidant activity is determined by the modified FRAP (Ferric Chloride reducing ability of Plasma) method. To 3.0 ml of FRAP reagent [2.5 ml of 0.3 M acetate buffer pH 3.6;0.25 ml of 10mM 2,4,6- tripyridyl –S –triazine (TPTZ) solution and 0.25 ml of 20mM ferric Chloride] , 0.1 ml of MSA (Methanol extract of S.aromaticum) , BHT and Quercetin at different concentrations are added and absorbance is measured at 595 nm. Blank is set up with 3.0 ml of FRAP regent and 0.1 ml of methanol and proceeded as per the test. The calibration curve was prepared using FeSO4 with concentrations ranging from 0-1mM. The results are expressed as Ascorbic acid Equivalent Antioxidant capacity (AEAC) in terms of mM.
DPPH Radical scavenging Activity
DPPH scavenging activity is measured by the method of Koleva et al ., (2002). To 1.0 ml of ethanolic solution of DPPH (0.3mM), 2.5ml of MSA,BHT and Quercetin at different concentrations are added. For control, test sample is replaced by methanol. The contents are incubated at 370C for 30 min and absorbance is measured at 517 nm using spectrophotometer. The percent inhibition of DPPH radical is calculated by the formula A0 – A x 100 / A0 where A0 is Absorbance of control and A is Absorbance of test sample. IC50 value denotes the concentration of sample, which is required to scavenge 50% of DPPH free radical.
Super oxide radical scavenging activity
The super oxide scavenging activity is measured by Beauchamp & Fridovich method (1971) with some modification. Superoxide anions are generated in a non- enzymatic hydroxyl amine(HA)-EDTA system and assayed by the reduction of nitroblue tetrazolium (NBT). The super oxide anion is generated in a reaction mixture containing 1.0 ml of sodium carbonate (125mM),0.4ml NBT(25mM) and 0.2 ml of EDTA(0.1mM),and 0.4 ml of hydroxyl amine (0.1mM). The reaction is initiated by adding 0.5 ml of different concentrations of MSA ,BHT and Quercetin to the mixture. After 5 min of incubation at room temperature, the absorbance at 560 nm is measured in spectrophotometer. The control is simultaneously run without MSA. The super oxide anion scavenging activity is calculated as percent inhibition of absorbance compared to the control.
Hydroxyl radical scavenging activity
The ability of the sample to inhibit hydroxyl radical mediated peroxidation is carried out according to method of Kunchandy & Rao (1990). The reaction mixture contained 0.1 ml of different concentration of MSA , BHT and Quercetin , 0.5 ml of 0.6mM deoxyribose in phosphate buffer (25mM, pH 7.4), 0.2 ml of premixed 0.02 mM ferrous ammonium sulfate and 0.02 mM EDTA (1:1 v/v) solution, 0.1 ml of ascorbic acid (0.6mM) and 0.1 ml of H2O2 (0.85mM) incubated at 370C for 15 min. After incubation, 1.5 ml of 2.8% cold TCA and 1.0ml of thiobarbituric acid (TBA) are added. The contents are vortexed and heated in a water bath at 500C for 15 min. The absorbance is measured at 532 nm. Control is set up without MSA. The percentage of inhibition values are calculated from the absorbance of the control (A0) and of the sample (A) using the formula, A0 – A x 100 / A0.
Hydrogen Peroxide scavenging activity
Hydrogen peroxide scavenging activity of the extract is estimated by the method of Zhang (2000). 1.0 ml of 0.1mM H2O2 and 1.0ml of various concentrations of MSA ,BHT and Quercetin are mixed, followed by 2 drops of 3% ammonium molybdate, 10 ml of 2M H2SO4 and 0.7 ml of 1.8M KI. The mixed solution is titrated with 5.09mM Na2S2O3 until yellow colour is disappeared. For control, all reagents are added except MSA. Percentage of scavenging of hydrogen peroxide is calculated as percent inhibition.
Nitric Oxide scavenging activity
Nitric oxide radical scavenging activity is determined according to the method reported by Garrat (1964). Sodium nitroprusside in aqueous solution at physiological pH spontaneously generates nitric oxide ions, which can be determined by the use of Griess IIIosvoy reaction. 2.0 ml of 10mM sodium nitroprusside in 0.5 ml phosphate buffer saline (pH 7.4) was mixed with 0.5 ml of MSA at various concentrations and the mixture is incubated at 250C for 150 min. From the incubation mixture 0.5 ml solution is taken out and added to 1.0 ml of sulfanillic acid reagent (33% in 20% glacial acetic acid) and incubated at room temperature for 5min. Finally 1.0ml of naphthylendiamine dihydrochloride (0.1w/v) is mixed and again incubated at room temperature for 30 min. The absorbance at 540nm is measured with a spectrophotometer. For control, methanol is used in place of MSA . The nitric oxide radical scavenging activity is calculated as percent inhibition.
Fe 2+ chelating activity
The chelating activity of MSA, BHT and Quercetin for ferrous ion is measured according to the method of Dinis et al., (1994). To 0.5 ml of methanol extract of S.aromaticum(MSA) at different concentrations, 1.6 ml of distilled water and 0.05ml of FeCl2 (2mM) are added. After 30 sec, 0.1 ml ferrozine (5mM) is added. Ferrozine reacts with the divalent iron to form stable magenta complex soluble in water. After 10 min at room temperature, the absorbance of the Fe2+-Ferrozine complex is measured at 562nm. Control is run simultaneously without MSA. The chelating activity of the extracts for Fe2+ is calculated as percent chelating rate using formula A0 – A x 100 / A0.
In vitro inhibition of lipid peroxidation
Lipid peroxidation induced by FeSO4-ascorbate system in sheep liver homogenate is estimated as thiobarbituric acid reacting substances (TBARS) by the method of Ohkawa et al., (1979). The reaction mixture contained 0.1 ml of sheep liver homogenate (25%) in Tris-HCl buffer (20mM pH 7.0) ; KCl (30mM) FeSO4 (NH4) SO4.7H2O (0.06mM)) and various concentrations of MSA , BHT and Quercetin in a final volume of 0.5 ml and incubated at 370C for 1hr. After incubation, 0.4 ml is removed and treated with 0.2ml of sodium dodecyl sulphate (8.1%), 1.5 ml of thiobarbituric acid (TBA) (0.8%) and 1.5 ml of trichloroacetic acid (20%). The total volume is made up to 4.0 ml with distilled water and then kept in a water bath at 95O C for 1hr. After cooling, 1.0ml of distilled water and 5.0 ml of n-butanol and pyridine mixture (15:1) are added to the reaction mixture, shaken vigorously and centrifuged at 4000 rpm for 10 min. The butanol pyridine layer is removed and its absorbance is measured at 532nm. Control is also run in the same manner but MSA is replaced with methanol. Inhibition of lipid peroxidation is determined by comparing the optical density (OD) of the MSA with that of the control.
Results and Discussion
The total antioxidant activity of methanol extract of S. aromaticum (MSA), synthetic antioxidant (BHT) and natural antioxidant (Quercetin) was determined and results are expressed in AEAC in terms of mM. The MSA showed significant total antioxidant activity with an AEAC value of 0.67mM which was higher than synthetic antioxidant BHT (0.48mM) and Quercetin (0.45mM) (Figure 1).
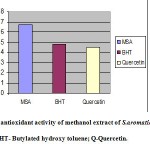 |
Figur :Total antioxidant activity of methanol extract of S.aromaticum –MSA; BHT- Butylated hydroxy toluene; Q-Quercetin.
|
DPPH is a stable free radical that accepts an electron of hydrogen radical to become a stable diamagnetic molecule. Hence DPPH is usually used as substrate to evaluate the antioxidant activity (Elmastas et al., 2006). The strength of the scavenging activity of methanol extract of S.aromaticum and standards on DPPH radical followed the order of MSA > BHT > Quercetin with percentage of inhibition of 91.72 ,80.13 and 68.48 at 1 mg / ml respectively. IC 50 values of S.aromaticum( MSA ) , BHT and Quercetin are 95,201,315 µg/ml respectively. These results indicated that MSA has a significant effect on scavenging free radicals. There is a direct proportionality of radical scavenging activity with concentration (Table 1). Based on the data obtained MSA is a potent free radical scavenging agent as well as primary antioxidant. Its activity against free radicals, may limit free radical damage occurring in human body.
Table 1: DPPH radical scavenging activity of methanol extract of S.aromaticum.
| Percentage of inhibition | |||
| Concentration(µg/ml) | S.aromaticum | Butylated hydroxy toluene | Quercetin |
| 10 | 30.12 ±0.18 | 28.20±0.230 | 20.14±0.30 |
| 50 | 38.12 ±0.16 | 36.16±0.30 | 27.08±0.18 |
| 100 | 43.18±0.20 | 42.12±0.41 | 33.16±0.22 |
| 250 | 55.40±0.22 | 52.02±0.20 | 44.20±0.21 |
| 500 | 62.43±0.32 | 57.60±0.30 | 55.33±0.22 |
| 700 | 70.45±0.20 | 65.20±0.30 | 61.26±0.31 |
| 1000 | 91.72±0.20 | 80.13±0.12 | 68.48±0.30 |
| C 50 | 95 | 201 | 315 |
Values are means ± SD (n= 5)
Superoxide anion is biologically important, since it decomposes to form stronger oxidative species such as single oxygen and hydroxyl radicals which are very harmful to the cellular components in a biological systems. Superoxide anion scavenging activity of MSA is shown in Table 2. The super oxide scavenging activity is proportional to the concentration of MSA.The half inhibition concentration IC50 value of MSA, BHT and Quercetin are 101, 105, 233 µg/ml respectively.
Table 2:Super oxide scavenging activity of methanol extract of S.aromaticum.
| Percentage of inhibition | |||
| Concentration(µg/ml) | S.aromaticum | Butylated hydroxy toluene | Quercetin |
| 10 | 09.06±0.02 | 08.02±0.02 | 07.09±0.02 |
| 50 | 22.23±0.04 | 20.24±0.01 | 19.11±0.03 |
| 100 | 40.30±0.03 | 36.11±0.04 | 32.11±0.04 |
| 250 | 61.42±0.04 | 60.42±0.02 | 49.42±0.02 |
| 500 | 77.63±0.02 | 66.55±0.04 | 62.06±0.01 |
| 700 | 81.22±0.03 | 75.25±0.01 | 70.48±0.01 |
| 1000 | 92.42±0.02 | 90.33±0.01 | 86.42±0.02 |
| C 50 | 101 | 105 | 233 |
Values are means ± SD (n= 5)
The hydroxyl radical is probably the final mediator of the free radical induced tissue damages. All of the reactive oxygen species exert most of their pathological effects by giving rise to hydroxyl radical formation. Table 3 showed that the MSA exhibited concentration dependent scavenging activities against hydroxyl radicals generated in Fenton reaction. The IC50 values of MSA, BHT and Quercetin are 250, 350 and 430 µg/ml respectively. These results reveal that MSA is exhibiting potent hydroxyl radical scavenging activity compared to BHT and Quercetin.
Table 3: Hydroxyl radical scavenging activity of methanol extract of S.aromaticum.
| Percentage of inhibition | |||
| Concentration(µg/ml) | S.aromaticum | Butylated hydroxy toluene | Quercetin |
| 10 | 07.12±0.011 | 05.27±0.032 | 04.28±0.072 |
| 50 | 20.05±0.032 | 18.08±0.038 | 17.39±0.062 |
| 100 | 32.12±0.044 | 30.17±0.042 | 29.12±0.052 |
| 250 | 48.63±0.037 | 46.25±0.048 | 45.18±0.011 |
| 500 | 69.62± 0.014 | 67.42±0.058 | 66.28±0.011 |
| 700 | 71.28±0.012 | 69.42±0.057 | 68.13±0.012 |
| 1000 | 85.41±0.015 | 83.38±0.020 | 82.22±0.013 |
| C 50 | 250 | 350 | 430 |
Values are means ± SD (n= 5)
The hydrogen peroxide scavenging ability of MSA is given in Table 4, where it is compared with that of BHT and Quercetin as standards. The MSA is exhibiting scavenging hydrogen peroxide in a concentration dependent manner. The correlation between the extract value and those of the control is statistically significant (p<0.05). The MSA, BHT and Quercetin exhibited hydrogen peroxide scavenging activity of 72.31, 70.62 and 68.28 %at 1 mg/ml with IC50 value of 225, 255 and 415 µg/ml respectively. Although hydrogen peroxide itself is not very reactive, it can sometimes cause cytotoxicity by giving rise to hydroxyl radicals in the cell. Thus decomposing hydrogen peroxide is very important in biological system.
Table 4: Hydrogen peroxide scavenging activity of methanol extract of S.aromaticum
| Percentage of inhibition | |||
| Concentration(µg/ml) | S.aromaticum | Butylated hydroxy toluene | Quercetin |
| 10 | 06.01±0.011 | 05.62±0.032 | 04.31±0.044 |
| 50 | 18.25±0.032 | 16.65±0.075 | 14.32±0.064 |
| 100 | 28.03±0.072 | 26.72±0.012 | 24.02±0.011 |
| 250 | 50.72±0.036 | 48.14±0.049 | 46.25±0.012 |
| 500 | 59.76±0.012 | 57.52±0.072 | 53.64±0.044 |
| 700 | 69.21±0.018 | 67.21±0.019 | 63.20±0.014 |
| 1000 | 72.31±0.016
|
70.62±0.015 | 68.28±0.022 |
| C 50 | 225 | 255 | 415 |
Values are means ± SD (n= 5)
Metal chelating activity influences the concentration of the catalyzing transition metal ions in lipid peroxidation. It has been reported that chelating agents are effective as secondary antioxidants because they reduce the redox potential, thereby stabilizing the oxidized form of the metal ion (Gordon, 1996). The data shown in the Table 5 indicates that the MSA demonstrate a significant iron binding capacity with IC50 values of 78µg/ml compared to known antioxidants(BHT 97µg/ml;Quercetin 205µg/ml) suggesting the protective action against peroxidation.
Table 5: Metal chelating activity of methanol extract of S.aromaticum.
| Percentage of inhibition | ||||
| Concentration(µg/ml) | S.aromaticum | Butylated hydroxy toluene | Quercetin | |
| 10 | 21.42±0.03 | 8.12±0.03 | 7.52±0.01 | |
| 50 | 36.12±0.03 | 32.42±0.05 | 28.41±0.01 | |
| 100 | 51.53±0.02 | 49.51±0.01 | 38.47±0.04 | |
| 250 | 62.06±0.02 | 61.43±0.03 | 59.62±0.05 | |
| 500 | 72.51±0.04 | 70.49±0.04 | 68.49±0.07 | |
| 700 | 82.42±0.01 | 79.12±0.02 | 72.12±0.04 | |
| 1000 | 90.58±0.01 | 89.62±0.01 | 86.62±0.06 | |
| C 50 | 78 | 97 | 205 | |
Values are means ± SD (n= 5)
Nitric oxide radicals and reactive nitrogen species are found to have biological roles in inflammation and in mediating many cytotoxic and pathological events (Darley et al., 1995). Methanol extract of S. aromaticum exhibited strong nitric oxide scavenging activity of 215 µg/ml in a dose dependent manner when compared to BHT(400 µg/ml) and Quercetin (425µg/ml ) (Table 6).
Table 6: Nitric oxide scavenging activity of methanol extract of S.aromaticum.
| Percentage of inhibition | |||
| Concentration(µg/ml) | S.aromaticum | Butylated hydroxy toluene | Quercetin |
| 10 | 03.01±0.032 | 2.88±0.032 | 2.35±0.064 |
| 50 | 15.72±0.011 | 13.97±0.084 | 7.47±0.044 |
| 100 | 26.03±0.072 | 23.15±0.015 | 15.72±0.011 |
| 250 | 46.84±0.044 | 45.52±0.056 | 43.25±0.012 |
| 500 | 63.48±0.037 | 57.13±0.068 | 55.84±0.044 |
| 700 | 72.31±0.012 | 61.21±0.014 | 60.21±0.013 |
| 1000 | 76.21±0.014 | 63.89±0.024 | 61.13± 0.022 |
| C 50 | 215 | 400 | 425 |
Values are means ± SD (n= 5)
Initiation of the lipid peroxidation takes place through hydroxyl radical by Fenton’s reaction. Figure 2 shows that the MSA inhibited FeSO4 induced lipid peroxidation of sheep liver homogenate in a dose dependent manner. The inhibition may be caused by scavenging the hydroxyl radical or superoxide radicals or by chelating the Fe+3 / Fe+2 or by reducing the rate of conversion of ferrous to ferric or by chelating the iron itself. The inhibition percentage of lipid peroxidation in the presence of MSA was found to be 87 % when compared to BHT and Quercetin (80 and 79%) at 1 mg /ml concentration.
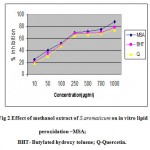 |
Figure: Effect of methanol extract of S.aromaticum on in vitro lipid peroxidation –MSA; BHT- Butylated hydroxy toluene; Q-Quercetin.
|
Conclusion
It has been concluded that the methanol extract of Syzygium aromaticum exhibits significant antioxidants activity through the scavenging of different free radicals such as superoxide, hydroxyl radical, hydrogen peroxide, nitric oxide and also iron chelation which participate in various pathophysiological conditions of diseases including ageing. Therefore the present study shows the MSA is a rich source of natural antioxidants that can be important in disease prevention, health preservation and promotion of longevity of life. Further studies are needed to isolate the bioactive components responsible for antioxidant activities.
References
- Khlifi, S., EI Hachimi ,Y., Khalil , A., ES- Safi ,N.,Belahyan, A., Tellal ,R.and EI Abbouyi, A. In vitro antioxidant properties of salvia verbenaca L.hydromethanolic extract. Indian J.pharmacol, 38 :276-280 (2006) .
- Halliwell, B . Antioxidant and human disease. A general introduction. Nutr Rev, 55 : 44-52( 1997).
- Brown, J.E . and Rice –Evans ,C.A. Luteolin rich artichoke extract protect low -density lipoprotein from oxidation .invitro. Free Radical Research, 29 :247-255 (1998).
- Liu, F.,Ooi, V.E.C. and Chang , S.T. Free radical scavenging activity of mushroom polysaccharide extracts. Life Science , 60: 763-771 (1997).
- Chopra, RN., Nayer, S.L.and Chopra, I.C. Glossary of Indian medicinal plants 3 rd edition. Council of Scientific and Industrial Research, New Delhi, 1992; India.
- Ahmad,I and Beg, A.Z. Antimicrobial and phytochemical studies of 45 Indian medicinal plants against multi –drug resistant human pathogen. J.Ethnopharmacol. 74 :113-123 (2001).
- Harborne, S.B. and Baxter, H. Phytochemical Dictionary. A handbook of bio-active compounds from plants Taylor and Francis, London. 1995.
- Abasta ,S.P. The useful plants of India. New Delhi: Publication and information Directorate, 1986 ; CSIR.
- Kwang-Geun lee and Takayuki shibamoto., Antioxidant property of aroma extract isolated from clove buds [ Syzigium aromaticum(L.) Merr.et perry]. Food chemistry, 74 ; 443 (2001).
- Steinmetz, K.A ., Potter, J.D ., Vegetables, fruits and cancer prevention: A review.J.Am. Diet.Association. 96: 1027 (1996).
- Koleva ,II., Van Beek ,T.A .,Linssen, J.P.H., De Groot ,A. and Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochemical analysis, 13:08-17 (2002).
- Beauchamp, C., Fridovich, I. Super oxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal.Biochem , 44:276.-287 (1971).
- Kunchandy, E and Rao, M.N.A.. Oxygen radical scavenging activity of curcumin. J. Pharmacogen, 58: 237-240 (1990).
- Zhang, X.Y.Principles of chemical analysis. Beijing: China Science Press. 275-276 (2000).
- Garrat, D.C. The quantitative analysis of drugs. Japan: Chapman and Hall. 3 :456–458 (1964).
- Dinis,TP.C., Madeira,V.M.C and Almeidam, L.M. Action of phenolic derivatives as inhibitors of membrane lipid peroxidation and peroxyl radical scavengers. Ach. Biochem. And Biophy. 315 :161-169 (1994).
- Ohkawa, H., Ohishi, N., Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction.Anal. Biochem, 95 :351-358 (1979).
- Elmastas, M., Gulcin ,I., Isildak, O., Kufrevioglu, O.I., Ibaoglu, K., Aboul-Enein ,H.Y.Radical scavenging activity and antioxidant capacity of Bay leaf extracts. J. of Iran. Chem. Society, 3:258-266 (2006).
- Gordon ,M.H. In: B.J.P. Hudson(ed), food antioxidants. Elsevier-London-New York. 1 (1990).
- Darley, Usmar ,V., Wiseman ,H., Nitric oxide and oxygen radicals: a question of balance. FEBS Lett.,369 :131-135 (1995).

This work is licensed under a Creative Commons Attribution 4.0 International License.





