Manuscript accepted on : September 17, 2010
Published online on: 28-12-2010
Subramanian Kumaresan1, Sanniyasi Elumalai2* and Mylsamy Prabhakaran2
1Department of Plant Biotechnology, Ramakrishna Mission Vivekananda College (Autonomous), Mylapore. Chennai - 600 004 India.
2PG and Research Department of Plant Biology and Plant Biotechnology, Presidency College (Autonomous), Triplicane, Chennai - 600 005 India.
Corresponding Author E-mail:ananandal@gmail.com
ABSTRACT: Mulberry, found in subtropical regions, is a deciduous plant with good nutritional value. Its leaves are thin, glossy and light green in color. The shape of the mulberry leaves could be quite different even on the same tree. Mulberry can balance internal secretions and enhance immunity. Presence of nutritious elements like minerals and vitamins in mulberry helps in controlling various diseases. Vesicular Arbuscular Mycorrhizas (VAM) fungi are the best known for their ability to improve plant growth in low phosphate soils by exploiting large areas and soil and actively transporting the phosphate back to plants. With respect to the properties of the fungi and the valuable property of leaf, made us to select two varieties of mulberry like MR2 and Kanva2. These two cultivars were inoculated with the six different strains of VAM fungi (Glomus microcarpum, Glomus aggregatum, Glomus fasciculatum, Glomus mosseae, Gigaspora margarita, Acaulospora scrobiculata). Various physiological parameters like micro nutrients, macro nutrients and various biochemical parameters were estimated which significantly implied that inoculated plants had greater concentration of the different organic compounds in the leaves when compared to uninoculated plants. Macro nutrient contents of nitrogen, phosphorus, potassium and the micro nutrients, zinc and copper significantly greater concentrations, except iron in leaves.
KEYWORDS: VAMF; Morus alba L; nutritional parameters; biochemical parameters
Download this article as:| Copy the following to cite this article: Kumaresan S, Elumalai S, Prabhakaran M. Effect of Vam Fungi on Growth and Physiological Parameters of Mulberry (Morus Alba L.) Cultivars in South India. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Kumaresan S, Elumalai S, Prabhakaran M. Effect of Vam Fungi on Growth and Physiological Parameters of Mulberry (Morus Alba L.) Cultivars in South India. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9287 |
Introduction
The term ‘Mycorrhizae’ was coined by Frank [6], for the mutualistic symbiosis between roots of vascular plants and certain fungi. The VAM fungi are present in practically all soils and associated with a great variety of plants of different taxonomic groups [11]. Several workers have reported the presence of VAM fungal spores in root-zone soils from different parts of India. Various species of Glomus, Sclerocystis, Gigaspora and Acaulospora in Indian soils have been reported [1, 23, 2, 22, 20, 10, 12, 21]
The mycorrhizae are vital for uptake and accumulation of ions from soil and translocation to host because of their high metabolic rate and strategically diffuse distribution in the upper soil layers [17]. In fact, the fungus serves as a highly efficient extension of the host root system. Minerals like N, P, Ca, S, Zn, Cu and Fe absorbed from soil mycorrhizal fungi and translocated to the host plant [24]. Minerals more than 4 cm distant from the nearest host root can be absorbed by the fungal hyphae and traslocated to roots in the mycorrhizal plants [25]. Bieleski [3] calculated that VAM fungi may increase the effective absorbing surface of the host root by as much as ten times.
Ions such as P, Zn and Cu do not diffuse readily through soil. Because of this poor diffusion, roots deplete the immobile soil nutrients from a zone immediately surrounding the root. Mycorrhizal fungal hyphae extend into the soil, penetrating the zone of nutrient depletion and can increase the effectiveness of absorption of immobile elements by as much as 60 times.
The mycorrizal fungi also produce enzymes, auxins, vitamins, cytokinins and other substances that increase rootlet size and longevity [4]. They to the host [16]. Two types of mycorrhizal fungal hyphae regulate nutrient movement, absorbing hyphae regulate nutrient movement, absorbing hyphae are fine, highly branched hyphae that explore substrates, absorbing nutrients released form adjacent soil or organic matter substances. In some instances, they also secrete enzymes capable of breaking down organic materials [8]. VAM fungi also alter the kinetic properties of the root, thereby changing its nutrient uptake abilities.
Materials and Methods
Nutritional Parameters
Fifth to seventh leaves from shoot tips of each treatment were oven dried at 40°C for three days. The temperature was gradually increased to 60°C and kept constant for two days. Acid digestion of the dried and powdered sample (500 mg) was carried out using 20 ml of triple acid mixture (HNO3 : HCl4 – 7:2:1) in a Kjeldahl flask. The colourless extract was transferred to a 100 ml volumetric flask and made up to 100 ml with distilled water. This sample was analysed for micro (Fe, Zn and Cu) and macro elements (N, P and K) at Tamil Nadu Rice Research Institute (TRRI), Aduthurai, Tamil Nadu.
The leaf nitrogen and potassium were determined by using microkjeldhal and flame photometric methods [9] respectively. The phosphorus content of leaf was determined colorimetrically by Vanadomolybdate phosphoric yellow colour method [9]. The micronutrients Fe, Zn and copper contents were measured in atomic absorbtion spectrophotometer [27].
Biochemical parameters
Fifth to seventh leaves from shoot tips of each treatment were collected and washed carefully in water to remove the adhering soil particles and dried. Reducing and total sugars, proteins, free amino acids and phenols were estimated in dried leaves, whereas the lipid content was estimated in fresh leaves and the known sample extracted with ethanol following the procedure outlined by Radhakrishnan et al. [18].
Reducing sugar
Reducing sugar was estimated with 10 ml of sample extracted in the 80% ethanol. The sample is treated with 1ml each of the following reagents (25% lead acetate, 25% sodium carbonate and Nelson- Somogi reagent). The sample mixture was heated at 100°C in a water bath for 20 minutes. After cooling the contents 1ml of arsenomolybdate was added and the total volume was made with distilled water. With glucose as standard reference, the optical density of the sample was measured at 540 nm [28].
Total soluble sugars
Total soluble sugars were calculated with 10 ml of sample extracted with 80% ethanol. Sample was mixed with 3ml of 1N HCl and kept in water bath for 20 minutes. Later it was cooled and neutralized with 3 ml of 1N NaOH, 1 ml of 25% lead acetate and 1 ml of 25% sodium carbonate. The sugar content was estimated by employing Nelson-Somogyi reaction as described above one. With glucose as standard reference the sample was measured for its optical density at 540 nm [28].
Total proteins
Total proteins were calculated using the sample extracted in distilled water. 0.5 ml of sample was subjected to 10% TCA precipitated which is centrifuged and the pellet is collected and dissolved in 0.1N NaOH. Later 0.5 ml of 0.1% copper sulphate, 2.5 ml of 12.5% sodium carbonate and 0.5 ml of 25% folinphenol reagent was added and mixed. The total volume was made up to 5ml and the reaction mixture was kept in dark for 30 minutes before measuring the optical density. Bovine serum albumin was taken as standard and the sample was read at 660 nm [15].
Total free amino acids
1 ml of the sample extracted using the 80% ethanol was treated with the 80 % of 1 ml phenol and placed in boiling water bath for 10 min. Later 0.2 ml of 5% ninhydrin is added and again kept in boiling water bath for 15 minutes. The total volume is made up to 10 ml using 60% ethanol. With Glycine as the reference the sample was read at 570 nm [26].
Total phenol
Leaf Sample extracted in 80% ethanol was used for calculating the total phenols. 1 ml of the sample was mixed with 1 ml of 20% sodium carbonate, 0.5 ml of folin phenol reagent and water in 1:2 dilutions and kept at 100°C in water bath for 10 min. The total known volume was made with distilled water. The sample was measured at 660 nm with catechol as standard reference [5].
Total lipids
The total lipids were calculated using the fresh leaves. 250 mg of sample is homogenized with the extract solvent (chloroform and methanol in 2:2 v/v). The contents were filtered through the filter paper. The filtrate collected is vortexed with the sodium sulphate. Then it was taken in a pre weighed beaker and dried by boiling. The dried extract is weighed by substrate in the initial weighed form. The final weight was expressed in µg/g-1 fresh wt [19].
Estimation of acid phosphatases in mulberry leaves
The cell free extract 0.5 ml is taken as sample. The sample is allowed to react with 0.5 ml of 10mM P-nitrophenyl phosphate (PNPP) in 0.1M acetate buffer pH 4 and 0.1 ml of 0.5mM of magnesium chloride. Later the contents were mixed and Incubated for 30 min. at 30°C. Reaction terminated by addition of 5 ml of 0.05 M NaOH. For control, no substrate was added. With P- nitrophenol as standard reference the sample was estimated at 405 nm (Gianinazzi-Pearson and Gianinazzi [7] modified by Krishna (1981).
Estimation of alkaline phosphatases in mulberry leaves
The cell free extract was taken as sample of about 0.5 ml treated with 0.9 ml of 10mM p-nitrophenyl phosphate (PNPP) in 0.05M Tris-citric acid buffer pH 8.5 and 0.1 ml of 0.5 mM MgCl2 were mixed and Incubated for 30 min. at 30°C. reaction terminated by addition of 5ml of 0.05M NaOH. For control, no substrate was added. P-nitophenol as the standard the sample was estimated at 405 nm using calorimetry. Gianinazzi-Pearson and Gianinazzi [7] modified by Krishna [13]. All colorimetric readings were taken using spectonic – 20 (Bauch and Lomb).
Determination of proteins qualitatively by SDS-PAGE (Sodium Dodecyl Sulphate-Poly Acrylamide Gel Electrophoresis) : (Laemmli, 1970[14] ).
Protein extraction
The extraction procedure was performed at 4°C. 50mg of fresh leaf samples were extracted with 0.5 M Tris buffer with pH 6.8. The suspension was filtered through cheese cloth and centrifuged at 5000 rpm in gravity centrifuge for 5 min. Then Ammonium sulphate was added (47.2 g/100ml W/V yielding a 70% standard solution) as fine powder to the supernatant and stirred for 15 min. to precipitated the protein and this was allowed to continued for further 30min. Then the mixture was centrifuged at 10,000 rpm for 40 min. at 4°C and then poured the supernatant out then resuspended the pellet (which can be unable and is not always visible) in 0.5 M tris buffer with pH 6.8, then the sample was subjected to SDS-PAGE.
SDS-PAGE Reagents
Solubilizing buffer
1 M Tris-HCl, pH 6.8 – 60 µl
B-Mercapto ethanol – 50 µl
10% SDS – 200 µl
100% glycerol – 100 µl
1mg ml-1 bromophenol blue – 1 µl
Separating gel buffer
4x Tris-HCl – 8.2 g
Distilled water – 60 ml
Adjusted the pH to 8.8 with 1N NaOH make the final volume into 100ml distilled water.
Staking gel buffer
4x Tris-HCl – 6.0 g
Distilled water – 80 ml
Adjusted the pH to 6.8 with 1N NaOH make up the final volume into 100ml with distilled water.
Acrylamide Stock
Acrylamide – 30.0 g
N’ Methelene bis acrylamide – 0.8 g
Distilled water – 100 ml
Electrode buffer (Stock) (or) Running buffer
5x Tris-HC – 15.1 g
Glycine – 72.0 g
SDS – 5.0 g
Distilled water – 1000 ml
Diluted the stock to 1x buffer, before used.
Protein stain
Dissolved 0.25% coomassie brilliant blue-R250 in 50% methanol and 7% v/v acetic acid.
Destaining Solution
Methanol – 50 ml
Acetic acid – 7 ml
Distilled water – 43 ml
Storing Solution
Methanol – 5.0 ml
Acetic acid – 7.5 ml
Distilled water – 87.5 ml
Standard proteins mixture: (Molecular weight markers)
Dissolved 1mg of following standard proteins in 1ml of 50mM NaCl2 – 1mM
sodium phosphate, pH 7.0 solution
Lactalbumins – 14.2 kDa
Carbonic anhydrase – 29.0 kDa
Albumin egg – 45.0 kDa
Bovine serum albumin – 66.0 kDa
The slab-gel unit was thoroughly cleared and dried it. The gel plate was fixed appropriate spacers on the gel marker. To avoid the leakage, vacuum grease was applied both sides of the spacers. The volume of the gel was measured using distilled water.
The separating gel solution was prepared (according to the volume required)
Reagent B – 11.25 ml
Reagent D – 18.00 ml
10% SDS – 0.20 ml
Distilled water – 15 ml
Degas the mixture and then added
10% Ammonium persulphat (APS) 150µl
NNN’N’-Tetra methyl ethylene diamine (TEMED) – 30 µl
The above solution was poured into the plate upto the level such that 3 cm gap was allowed for staking gel. The air bubbles were removed and then added even layer of isobutanol on the top of the separating solution, to get a flat surface on the top of the gel. Then allowed it to polymerized for 30min. Then the isobutanol layer was removed and washed it with water.
Then the stacking gel solution was prepared (according to the volume required) by mixing of:
Reagent B – 3.0 ml
Reagent C – 5.0 ml
10% SDS – 0.2 ml
Distilled water – 12.0 ml
Degas the mixture and then added
APS -100 µl
TEMED – 20 µl
Then the comb was inserted in between the plates. The solution was poured carefully on the top of the separating gel and bubbles were removed. After 20 min. the bottom spacer was removed and the gel plate fixed with slab gel unit. The comb was removed and filled the well with running buffer (e). equal volume of solubilizing buffer was added to the sample solution and boiled in a water bath for 3 min. then about 300µl of above sample mixture was loaded into each well and instead of sample the equal amount of standard protein mixture was loaded to any one of the well to compared the molecular weight of the sample proteins. Then running buffer was added in anode and cathode chambers until the buffer touches the gel. The power supply was connected and applied 30V until the marker dye enter the separating gel and then increased the voltage 40 to 50. The power supply was continued until the marker dye reached the bottom of the gel. After reaching the marker dye, the power supply was disconnected and removed the slab gel set up. Then the glass plate was removed and placed the gel in coomassie brilliant blue R250 stain (f) for 2 to 4 hours. Then the gel was destained in destaining solution (g) until clear back ground was obtained and the gel was stored in solution mixture (h).
Results and Discussion
A comparison of protein content and patterns in leaf extracts from non-mycorrhizal and VAM inoculated mulberry cultivar MR2 plants has been made. Soluble protein content was higher in mycorrhizal than non-mychorrhizal leaves (Figure – 1). Electrophoretic analysis of ‘Tris-HCl’ protein extract on SDS-PAGE at 10% acrylamide showed that 4 to 7 polypeptides ranged between 10.0-39.0 kDa were expressed in six native VAM fungi inoculated and non-inoculated mulberry cultivar MR2. Several additional polypeptides appeared in mycorrhizal extract (Fig.1. A). after separation by electrophoretic analysis, the molecular weights 14.0, 17.0, 29.0 and 32.0 kDa polypeptides were expressed in all the VAM treated mulberry leaves. Polypeptide 19 kDa was expressed in Glomus mosseae, G. fasciculatum and Gigaspora margarita inoculated plants, while 39.0 kDa was expressed in Glomus aggregatum, G. mosseae and G. fasciculatum inoculated mulberry leaves.
A comparison of protein content and patterns in leaf extracts from non-mycorrhizal and native VAMF inoculated mulberry cultivar Kanva2 plants has been made. Soluble protein content was higher in mycorrhizal than non-mycorrhizal (Fig.1. B). After electrophoretic separation, several polypeptides appeared ranged between 14.0-35.0 kDa were expressed in mycorrhizal and non-mycorrihizal cultivar leaves. The molecular weight 14.0, 17.0, 21.0, and 32.0 kDa were expressed in all treatments, whereas 26.0, 32.0 and 35.0 kDa in all VAMF inoculated plants and 19.0 and 21 kDa in Glomus fasciculatum and dually inoculation of Glomus aggregatum and G. fasciculatum.
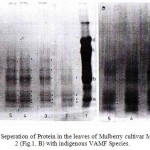 |
Figure 1: Electrophoretic Seperation of Protein in the leaves of Mulberry cultivar MR2 (Fig.1. A) and Kanva 2 (Fig.1. B) with indigenous VAMF Species
|
The results of the macro and micro nutrient contents in the leaves of MR2 cultivar was presented in table – 1. Mulberry cultivar MR2 plants inoculated with six species of native VAM fungi were compared with uninoculated mulberry cultivar, for macro nutrient contents of nitrogen, phosphors, potassium and the micro nutrients, zinc and copper significantly greater concentrations, except iron in leaves. These apparently were also significantly differences among the six VAM fungi (Table – 2). Glomus microcarpum seemed consistently more efficient in nutrient uptake particularly P, K and Zn with the host plant followed by Glomus fasciculatum and G.aggregatum. Glomus aggregatum seemed consistently more efficient in N uptake with host plant at 120th day.
Table 1: Effect of different native VAM fungal species on nutrient contents of mulberry cultivar MR2.
|
Period |
Treat ment |
Macro nutrients (%) | Micro nutrients (ppm) | ||||
| Nitrogen | Phosphorus | Potassium | Iron | Copper | Zinc | ||
|
60th day
90th day
120th day |
T1
T2 T3 T4 T5 T6 T7 T1 T2 T3 T4 T5 T6 T7 T1 T2 T3 T4 T5 T6 T7 |
2.3±0.02 a
3.1±0.03 c 2.9±0.02 b 3.2±0.03 c 2.8±0.02 b 3.1±0.02 c 2.7±0.02 b 2.6±0.02 a 3.9±0.06 c 3.0±0.06 c 3.6±0.06 b 3.1±0.04 b 2.7±0.05 ab 2.9±0.02 b 2.4±0.02 ab 3.6±0.02 d 2.9±0.03 c 3.5±0.04 d 3.2+0.04 c 2.5±0.02 b 3.0±0.02 c |
0.170±0.03 a
0.180±0.02 d 0.155±0.01 b 0.210±0.02 d 0.165±0.01 c 0.170±0.02 c 0.175±0.01 c 0.160±0.01 a 0.290±0.01 c 0.230±0.02 b 0.275±0.03 b 0.225±0.02 b 0.230±0.03 b 0.230±0.03 b 0.155±0.01 a 0.245±0.02 c 0.220±0.02 b 0.255±0.01 c 0.320±0.02 d 0.220±0.01 b 0.230±0.01bc |
2.8±0.04 b
2.5±0.04 a 3.1±0.05 c 2.4±0.05 a 2.7±0.02 ab 3.0±0.04 c 2.7±0.05 ab 2.6±0.02 a 2.9±0.88 b 3.2±0.06 c 2.9±0.08 b 3.3±0.10 c 3.2+0.10 c 2.8+0.10 b 2.2±0.01 a 2.8±0.20 b 2.5±0.10 ab 2.7±0.08 b 3.1±0.04 bc 3.0±0.03 bc 2.7±0.02 b |
316±1.9 b
318±1.8 b 396±1.0 c 312±1.2 a 282±1.2 a 276±0.9 a 276±0.9 a 320±0.8 a 344±0.8 d 394±1.1 e 396±1.4 e 340±1.4 d 332±1.3 c 328±1.1 b 332±1.1 b 352±1.1 c 360±1.3 c 390±1.4 e 364±0.9 d 324±0.7 a 328±0.7 b |
4±0.02 b
4±0.02 a 6±0.03 b 6±0.02 b 4±0.01 a 6±0.03 b 6±0.03 b 6±0.02 a 8±0.10 b 8±0.10 b 12±0.10 d 12±0.1 d 10±0.10 c 10±0.08 c 10±0.10 a 10±0.10 a 14±0.12 b 16±0.1 b 18±0.08 c 10±0.06 a 10±0.06 a |
32±0.7 c
26±0.7 d 24±0.7 a 32±0.4 c 44±0.4 d 30±0.3 c 22±0.4 a 44±0.4 a 52±0.4 ab 56±0.8 b 64±0.7 c 82±0.8 d 60+0.3 c 44±0.4 a 40±0.4 a 48±0.7 b 56±0.8 c 68±0.7 d 86±0.8 e 62±0.9 d 42±0.9 a |
Note: T1 – Control (non-mycorrhiza),
T5 – Glomus microcarpum (Thhiruvananthapuram isolate)
T2 – Glomus aggregatum (Idukki isolate),
T6 – Acaulospora scrobiculata (Malapuram isolate ) T3 – Glomus mosseae (Kottayam isolate),
T7 – Gigaspora margarita (Alapuzha isolate)
T4 – Glomus fasciculatum (Waynad Isolate),
Mean in each column followed by the same letter are not significantly different (p < 0.05) from each other according to DMR test.
Table 2: ANOVA (mean square) for biochemical and nutrient parameters in mulberry cultivar MR2, with seven treatments.
|
Parameters |
60th day |
90th day |
120th day |
|||||||||
| Treat-
ment |
Error | F. value | Sig* | Treat-
ment |
Error | F. value | Sig* | Treat-
ment |
Error
|
F. value
|
Sig*
|
|
| Chlorophyll a
Chlorophyll b Total Chlorophyll Reducing sugar Total sugar Protein Free amino acid Phenol Nitrogen Phosphorus Potassium |
0.004
0.010 0.020 132.516 277.250 14.821 7.622 2.504 2.068 0.007 1.526 |
0.006
0.007 0.018 42.558 67.986 3.285 8.401 5.114 1.539 0.018 3.429 |
1.432
3.525 2.599 7.265 9.515 10.589 2.117 1.143 4.703 1.431 1.558 |
0.271
0.024 0.066 0.001 0.000 0.000 0.116 0.389 0.004 0.250 0.209 |
0.431
0.592 1.592 253.555 1482.234 23.355 279.681 8.892 4.719 0.044 2.243 |
0.019
0.633 0.053 60.868 875.618 105.519 4.258 1.121 1.080 0.017 1.834 |
51.547
2.179 69.937 9.719 3.949 5.248 153.231 18.513 15.297 9.333 4.280 |
0.000
0.107 0.000 0.003 0.016 0.005 0.000 0.000 0.000 0.000 0.006 |
1.271
0.156 21.127 434.996 1804.450 353.328 606.535 18.338 5.491 0.060 2.372 |
0.223
0.185 0.833 47.379 44.049 17.705 12.927 60.581 1.481 0.030 2.443 |
14.248
2.106 6.384 21.423 95.585 46.574 109.491 6.502 12.981 6.957 3.399 |
0.0000
0.1134 0.0017 0.0000 0.0000 0.0000 0.0000 0.0019 0.0000 0.0004 0.0167 |
Note : *Significant at 0.05%
The results of the macro and micro nutrients in leaves of Kanva2 cultivar are given in the Tables – 3 & 4. Mulberry cultivar Kanva2 inoculated with native and introduced VAM fungi were compared with uninoculated mulberry cultivar, macro nutrient contents of N, P, K and micro nutrients, Fe, Cu and Zn significantly greater concentration. These apparently were also significant differences among these two VAM fungi (Table – 3 & 4). Dual inoculation of Glomus aggregatum and Glomus fasciculatum plants seemed consistently more efficient in nutrient uptake particularly N and P and micro nutrients Fe, Cu and Zn. Glomus aggregatum seemed consistently more efficient in N and K with host plant at 120th day, whereas in introduced VAM fungi treated plants, dual inoculation of Glomus aggregatum and Glomus fasciculatum seemed consistently more efficient in macro and micro nutrient contents.
Table 3: Effect of different native VAM fungal species on nutrient contents in mulberry cultivar Kanva 2
| Period | Treat ment | Macro nutrients (%) | Micro nutrients (ppm) | ||||
| Nitrogen | Phosphorus | Potassium | Iron | Copper | Zinc | ||
|
60th day
90th day
120th day |
T1
T2 T3 T4 T1 T2 T3 T4 T1 T2 T3 T4 |
1.75±0.06 a
1.99±0.04 b 2.43±0.20 c 2.55±0.20 c 2.6±0.10 a 3.11±0.20 b 3.56±0.20 c 3.12±0.30 b 2.37±0.10 a 2.99±0.10 b 3.50±0.30 c 2.86±0.20 b |
0.200±0.02 a
0.301±0.01 c 0.270±0.03 b 0.321±0.04 c 0.220±0.03 a 0.345±0.02 b 0.365±0.03 c 0.395±0.02 c 0.210±0.03 a 0.320±0.04 b 0.370±0.04 c 0.415±0.03 d |
2.7±0.1 a
3.4±0.2 c 3.1±0.1 b 3.0±0.2 b 2.8±0.1 a 3.9±0.2 c 3.9±0.1 c 3.4±0.1 b 3.1±0.1 a 3.7±0.1 b 3.7±0.2 b 3.3±0.1 c |
710±3.1 a
710±4.2 a 714±3.1 b 716±3.0 b 840±3.0 a 864±3.0 b 862±3.1 b 878±4.0 c 880±4.0 a 886±4.1 b 886±4.1 b 892±3.0 c |
14±0.01 b
12±0.01 a 14±0.02 b 14±0.01 b 14±0.03 a 16±0.03 b 16±0.01 b 18±0.01 c 18±0.02 a 22±0.02 c 20±0.01 b 24±0.01 b |
126±0.8 b
90±0.9 a 96±0.9 a 132±0.9 b 124±1.0 a 132±1.1 b 134±1.1 c 136±1.2 c 122±1.1 a 128±1.1 b 132±1.0 c 136±1.3 c |
Note: T1 – Control (non-mycorrhiza),
T2 – Glomus aggregatum (Idukki isolate),
T3 – Glomus fasciculatum (Waynad Isolate),
T4 – Glomus aggregatum and Glomus fasciculatum (nativeIsolate),
Mean in each column followed by the same letter are not significantly different (p < 0.05) from each other according to DMR test.
Table 4: Effect of different introduced VAMF species on nutrient contents in mulberry cultivar Kanva 2.
| Period | Treat ment | Macro nutrients (%) | Micro nutrients (ppm) | ||||
| Nitrogen | Phosphorus | Potassium | Iron | Copper | Zinc | ||
|
60th day
90th day
120th dat |
T1
T2 T3 T4 T1 T2 T3 T4 T1 T2 T3 T4 |
1.83±0.2 a
2.16±0.2 a 2.66±0.2 b 2.82±0.2 c 2.54±0.2 a 2.29±0.3 b 3.64±0.3 bc 4.25±0.2 c 2.62±0.2 a 3.10±0.1 a 3.62±0.2 b 4.04±0.2 b |
0.20±0.01 a
0.31±0.02 b 0.31±0.03 b 0.39±0.03 c 0.23±0.03 a 0.43±0.06 b 0.48±0.03 b 0.53±0.03 b 0.22±0.03 a 0.43±0.03 b 0.50±0.02 b 0.49±0.01 b |
3.09±0.3 a
3.41±0.4 a 3.43±0.3 a 3.73±0.3 a 3.57±0.3 a 4.14±0.5 a 3.86±0.3 b 4.31±0.3 a 3.68±0.3 a 3.97±0.3 a 4.01±0.2 a 4.42±0.3 c |
428±2.0 a
556±2.1 c 560±2.1 c 498±1.9 b 608±2.3 a 612±2.3 a 618±2.1 b 608±2.0 d 612±3.0 a 618±3.0 b 800±2.4 c 616±2.1 b |
18±0.01 b
12±0.03 a 20±0.03 c 12±0.01 a 16±0.01 b 14±0.01 a 22±0.10 c 14±0.02 a 18±0.02 a 22±0.02 b 22±0.01 b 18±0.01 a |
84±0.1 a
86±0.1 a 86±0.1 a 76±0.1 a 88±0.2 b 90±0.03 b 90±0.6 b 80±0.2 a 86±0.9 b 90±0.9 b 90±0.9 b 80±0.5 a |
Note: T1 – Control (non-mycorrhiza),
T2 – Glomus aggregatum (Bangalore isolate),
T3 – Glomus fasciculatum (Rotham Isolate),
T4 – Glomus aggregatum and Glomus fasciculatum (introduced isolate),
Mean in each column followed by the same letter are not significantly different (p < 0.05) from each other according to DMR test.
The chlorophyll contents per gram fresh weight of leaves was maximum plants inoculated with Glomus microcarpum and minimum in G. mosseae (Table – 5). Mulberry cultivar MR2 plants responded best to inoculation with Glomus microcarpum followed by G. fasciculatum and G. aggregatum.
Table 5: Influence of different native VAM fungi on chlorophyll contents in mulberry cultivar-MR2.
|
Treatment |
60th day (µg/mg) | 90th day (µg/mg) | 120th day (µg/mg) | ||||||
| Chloro
phyll a |
Chloro
phyll b |
Total Chloro
Phyll |
Chloro
phyll a |
Chloro
phyll b |
Total Chloro
Phyll |
Chloro
phyll a |
Chloro
phyll b |
Total Chloro
Phyll |
|
| T1
T2 T3 T4 T5 T6 T7 |
0.80±0.01 ab
0.81±0.01 b 0.80±0.03 ab 0.79±0.14 ab 0.78±0.01ab 0.76±0.01 a 0.78±0.00 ab |
0.56±0.01 b
0.58±0.03 abc 0.61±0.01 c 0.59±0.01 c 0.54±0.01 a 0.56±0.01 ab 0.58±0.01 ab |
1.38±0.03 bc
1.40±0.03 bc 1.43±0.02 c 1.39±0.02 abc 1.35±0.01 ab 1.32±0.02 a 1.35±0.01 ab |
0.69±0.00 a
0.99±0.04 c 0.89±0.01 b 1.11±0.02 d 1.10±0.02 b 1.01±0.03 c 1.16±0.02 e |
0.56±0.02 a
1.08±0.32 b 0.70±0.02 ab 0.82±0.01 ab 0.80±0.03 ab 0.83±0.03 ab 1.05±0.02 b |
1.27±0.03 a
1.75±0.04 c 1.61±0.02 b 1.91±0.03 d 1.85±0.09 cd 1.85±0.05 cd 2.23±0.03 e |
0.80±0.04 a
1.10±0.08 b 1.01±0.07 b 1.43±0.03 c 1.53±0.07 c 1.05±0.04 b 1.24±0.20 b |
0.71±0.02 a
0.85±0.07 a 0.72±0.05 a 0.92±0.12 b 0.93±0.11 b 0.84±0.03 a 0.82±0.05 a |
1.52±0.05 a
1.96±0.15 b 1.75±0.20 a 2.28±0.10 cd 2.47±0.21 d 2.19±0.26 cd 1.98±0.11 b |
Note: T1- Control (Non-mycorrihzal)
T4-Glomus fasciculatum (Wayanad isolate)
T2- Glomus aggregatum (Idukki isolate)
T5-Glomus microcarpu (Thruvananthapuram isolate)
T3- Glomus mosseae (Kottayam isolate)
T6- Acaulospora scrobiculata (Malapuram isolate) ,
T7- Gigaspora margarita (Alappuzha isolate)
Mean in each column followed by the same letters are not significantly different (P<0.05) from each other according to DMR test.
The chlorophyll contents per gram fresh weight of leaves was maximum in plants inoculated with Glomus aggregatum at 60 and 90th days and dual inoculation treated plants at 120 day followed by Glomus faciculatum (Table – 6), whereas in introduced VAMF both inoculated plants showed increased chlorophyll contents at all the time intervals (Table – 7).
Table 6: Effect of different native VAMF species on chlorophyll contents in mulberry cultivar Kanva2.
| Period | Treatment | Chlorophyll a
(µg/mg) |
Chlorophyll b
(µg/mg) |
Chlorophyll c
(µg/mg) |
|
60th day
90th day
120th day |
T1
T2 T3 T4 T1 T2 T3 T4 T1 T2 T3 T4 |
1.54±0.02 a
1.60±0.07 a 1.65±0.10 a 1.51±0.07 a 1.45±0.12 b 1.64±0.05 b 1.57±0.02 b 0.91±0.06 a 1.48±0.09 ab 1.60±0.03 b 1.78±0.02 c 1.37±0.01 a |
0.83±0.08 a
0.87±0.09 a 0.85±0.1 a 0.81±0.09 a 1.12±0.14 ab 1.16±0.16 ab 0.91±0.03 a 1.39±0.04 b 1.28±0.22 a 1.16±0.74 a 1.21±0.04 a 1.23±0.05 a |
2.40±0.08 a
2.48±0.02 a 2.51±0.11 a 2.36±0.09 a 2.65±0.25 ab 2.83±0.13 b 2.49±0.05 ab 2.21±0.09 a 2.55±0.16 a 2.85±0.10 ab 2.56±0.79 a 3.35±0.28 b |
Note : T1 – Control,
T2 – Glomus aggregatum (Idukki isolate),
T3 – Glomus fasciculatum (Waynad isolate),
T4 – Glomus aggregatum and Glomus fasciulatum (Native isolate).
Mean in each column followed by the same letters are not significant different (P<0.05) from each other according to DMR test.
Table 7: Effect of different introduced VAMF species on chlorophyll contents in mulberry cultivar Kanva 2.
|
Period |
Treatment |
Chlorophyll a
(µg/mg) |
Chlorophyll b
(µg/mg) |
Chlorophyll c
(µg/mg) |
|
60th day
90th day
120th day |
T1
T2 T3 T4 T1 T2 T3 T4 T1 T2 T3 T4 |
1.36±0.07 a
1.54±0.08 a 1.65±0.17 a 1.64±0.09 a 1.44±0.08 a 1.66±0.09 ab 1.85±0.13 b 1.93±0.08 b 1.31±0.06 a 1.61±0.04 b 1.70±0.13 b 1.83±0.08 b |
1.11±0.05 a
0.30±0.08 ab 1.38±0.14 ab 1.41±0.08 b 1.22±0.08 a 1.44±0.08 ab 1.59±0.12 b 1.70±0.12 b 1.12±0.07 a 1.33±0.06 ab 1.44±0.12 b 1.60±0.08 b |
2.47±0.13 a
2.69±0.17 ab 3.18±0.26 a 3.15±0.14 b 2.71±0.18 a 3.15±0.17 ab 3.58±2.24 b 3.65±0.21 b 2.45±0.12 a 3.01±0.10 b 3.23±0.26 a 3.43±0.17 b |
Note : T1 – Control (non-mycorhizal),
T2 – Glomus aggregatum (Bangalore isolate),
T3 – Glomus fasciculatum (Rothamsted isolate),
T4 – Glomus aggregatum and Glomus fasciculatum (Introduced isolate)
Mean in each column followed by the same letters are not significant different (P<0.05) from each other according to DMR test.
The increase in dry matter content could only be due to increase in organic matter content of the plant. The effectiveness of an endophyte depend mainly on the physiological and biochemical nature of the fungus. In general, inoculated plants had greater concentration of the different organic compounds in the leaves compared to uninoculated plants. Plants inoculated with Glomus aggregatum showed increase in reducing sugar, total proteins, amino acids and phenols studied followed by Glomus fasciculatum. The increase in total soluble sugars was maximal in Glomus mosseae inoculated plants, while acid and alkaline phosphatases was significantly greater in Glomus microcarpum compared to uninoculated plants. The lipid contents was more pronounced in plants inoculated with Glomus fasciculatum followed by G. microcarpum .
The results of the biochemical parameters in leaves of Kanva2 inoculated with native and introduced VAMF species; In general, inoculated plants had greater concentration of the different organic compounds in the leaves when compared to uninoculated plants. Plants inoculated with both cultures of Glomus aggregatum and Glomus fasciculatum showed increase in reducing and total sugars, total proteins, amino acids, lipids, phenols, acid and alkaline phosphatases studied followed by single inoculation of Glomus fasciculatum and G. aggregatum.
Conclusions
Vesicular Arbuscular Mycorrhizas (VAM) fungi are the best known for their ability to improve plant growth in low phosphate soils by exploiting large areas and soil and actively transporting the phosphate back to plants. With respect to the properties of the fungi and the valuable property of leaf, made us to select two varieties of mulberry like MR2 and Kanva2. These two cultivars were inoculated with the six different strains of VAM fungi (Glomus microcarpum, Glomus aggregatum, Glomus fasciculatum, Glomus mosseae, Gigaspora margarita, Acaulospora scrobiculata). Various physiological parameters like micro nutrients, macro nutrients and various biochemical parameters were estimated which significantly implied that inoculated plants had greater concentration of the different organic compounds in the leaves when compared to uninoculated plants. Macro nutrient contents of nitrogen, phosphorus, potassium and the micro nutrients, zinc and copper significantly greater concentrations, except iron in leaves.
Acknowledgements
The author would like to thank the Principal and Head of Plant Biotechnology, Ramakrishna Mission Vivekananda College (Autonomous), Mylapore, Chennai-600 004, Tamil Nadu, India and Principal and Head of Plant Biotechnology, Presidency College (Autonomous), Triplicane, Chennai-600 005, Tamil Nadu, India.
References
- Battacharjee, M. and Mukerji, K.G., Studies on Indian Endogonaceae II, The genus Glomus, Sydowia. 33: 14 (1980).
- Battacharjee, M., Mukerji, K.G. and Tewari, W. Skorpad., Structure and hyperparasitism of a new species of Gigaspora, Trans. Br. Mycol. Soc., 78: 184 (1982).
- Bielski, R.L., Phosphate pools, phosphate transport and phosphate availability, Rev.pl.physiol., 24: 225 (1973).
- Dixon, R.K., Cytokinin activities in Citrus jambhiri seedlings colonized by vesicular-arbuscular mycorrhizal fungi. (eds.) A. Mahadevan, N. Raman and K. Natarajan, Proc. First Asian Conference of Mycorrhizae. University of Madras, India.136 (1988).
- Farkas, G.L. and Kiraly, Z., Role of phenolic compounds in the physiology of plant disease and disease resistance. 2(4): 105 (1962).
- Frank, A.B., Nene Mitteilungen uber die Mycorrhizader Baume and dex Monotropa hypopitys, Ber. Deeut. Bot. Ges., 3: 27 (1885).
- Gianiazzi-Pearson, V. and Gianinazzi, S., Enzymatic studies on the metabolism of vesicular-arbuscular mycorrhiza. I. Effect of mycorrhiza formation and phosphorus nutrition on soluble phosphatase activities in onion roots, Veg.14: 833 (1976).
- Haselwandtor, K., Mycorrhizal infection and its possible ecological significance in climatically and nutritionally stressed on plant communities. Bot. 61: 107 (1987).
- Jackson, M.L., Soil Chemical Analyses, Prentice Hall, New Delhi. 111(1973).
- Janardhanan, K.K., Abdul-Khaliq. Fauzia Naushin. and Ramaswamy, K., Vesicular arbuscular mycorrhiza in an alkaline Usar land ecosystem, Curr. Sci. 67: 465 (1994).
- Jeffries, P., Use of mycorrhiza in agriculture, Rev. in Biotech. 5: 319 (1987).
- Joshi, K.C. and Sing, H.P., Interrelationships among vesicular-arbuscular mycorrhizae, population, soil properties and root colonization capacity of soil, Indian. Soc. of. Soil Sci. 43(2): 204 (1995).
- Krishna, K.R., Studies on mechanism of improved plant growth due to vesicular-arbuscular mycorrhiza, Ph.D. Thesis, University of Agril. Sciences, Bangalore.139 (1981).
- Laemmli, U.K., Cleavage of structural proteins during the assembly of the head of bacteriophage T4, , 227 (1970).
- Lowri, O.H., Rose Brough, N.J., Farr, A.L. and Randall, R.J., Protein estimation with folin-phenol reagent, J. Biol. Chem. 193: 265-275 (1951).
- Mexal, J. and Reid, C. P. P., The growth of selected mycorrhizal fungi response to induced water stress, J. Bot., 51: 1579 (1973).
- Mukerji, K.G., Bhattacharje and Mohan, M., Ecology of the Indian Endogonaceae, Bot., 56: 121 (1982).
- Radhakrishnan, A.N., Vaidyanathan, C.S. and Giri, K.V., Nitrogen constituents in plants. I. Free aminoacid in leaves and leguminous seeds, Indian Inst. Sci., 37: 178(1955).
- Sato, N. and Murata, N., Membrane lipids. In: Methods in Enzymolgy, (eds.) L. Packer and A.N. Glazer. 251(1988).
- Selvaraj, T. and Subramanian. G., Light and scanning electron microscope studies of vesicular-arbuscular mycorrhizal fungi in Sesamum indicum var. Co-1 roots. In: Mycorrhizae for Green Asia, (eds) A. Mahadevan, N. Raman and K. Nararajaj. Proc. First Asian Conference on Mycorrhizae, University of Madras, India.106 (1988).
- Selvaraj, T., Kalaivendan., C. and Bhaskaran, C., Influence of different inocula of vesicular arbuscular mycorrhizal fungi on growth, organic compounds and nutrition of Physalis minima Acta Bot. Indica, 230: 99 (1995).
- , S.K., Sharma., G.D. and Mishra, R.R., Status of mycorrhizae in sub-tropical forest ecosystem of Meghalaya, Acta Bot. Indica.14: 87 (1986).
- , K. and Varma, A.K., Endogonaceous spores associated with xerophytic plants in northern India, Trans. Br. Mycol. Soc. 77: 655 (1981).
- , S.E., Dickson, S., Morris, C. and Smith. F.A., Transfer of phosphate from fungus to plant in VA-mycorrhizas. Calculation of the area of symbiotic interface and of fluxes of P from two different fungi to Allium porrum L. New Phytol. 127: 93 (1994).
- Trappe, J.M., Mycorrhizae and productivity of arid and semiarid rangelands, In: Advances in food producing systems for arid and semiarid rangelands, J. T. Manassah and E. J. Brishey. Academic Press, Newyork. 581 (1981).
- Troll, W. and Canan., K., A modified photometric ninhydrin method for the analysis of amino-imino acids, Biol.Chem. 200: 803 (1953).
- Ure, A.M., Atomic absorption and flame emission spectrometry. In: Soil analysis: Instrumental techniques and related procedures, (eds.) K. A. Smith and M. Dekker. Inc., New York. 1: 114 (1983).
- Warten, D.C. and Mc Carty., Determination of glucose. In: Expeiments and methods in Biochemistry, Mac Millan Co, New York. 196(1972).

This work is licensed under a Creative Commons Attribution 4.0 International License.





