Manuscript accepted on : May 21, 2010
Published online on: 28-12-2010
B. Hassine Dorsaf1,2, Ben Ismail Hanen2, Jribi Chokri1, Khouja Mohamed Larbi3 and Abderrabba Manef1
1Unite de Recherche Physicochimie mol?culaire, IPEST, La Marsa Tunisie.
2Laboratoire de Technologie Alimentaire, Institut National Agronomique de Tunisie, 4 3 Avenue Charles Nicolle, 1082, Tunis Tunisie.
3Laboratoire Ecologie et Am?lioration Sylvo- Pastorale, INRGREF, B.P 10, Ariana 2080 Tunisie.
ABSTRACT: In this study we have determined the yield of the essential oil of 15 Eucalyptus species collected from Sidi Ismail Arboretum, in the Sahel of Tunisia. The average yield of leaf essential oil obtained by hydrodistillation varies according to species. It ranged between 3.367 % for E. dumosa and 1.167% for E. bicolor. The volatile oil compositions of E. dumosa, E. cladocalyx, E. oleosa and E. transcontinentalis were analysed qualitatively and quantitatively by GC/MS. Forty six different components were separated, and most of them identified. The main constituent was found to be 1-8 cineol (Eucalyptol). The extract possessed high yield of oxygenated monoterpens, followed by monoterpens hydrocarbons, then a wick level of sesquiterpens hydrocarbons.
KEYWORDS: Myrtaceae; Eucalyptus essential oil; GC/MS; 1-8cineol; oxygenated monoterpens
Download this article as:| Copy the following to cite this article: Dorsaf B. H, Hanen B. I, Chokri J, Larbi K. M, Manef A. Chemical Composition of Some Tunisian Eucalyptus Essential Oils As Obtained By Hydrodistillation Using Clevenger Type Apparatus. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Dorsaf B. H, Hanen B. I, Chokri J, Larbi K. M, Manef A. Chemical Composition of Some Tunisian Eucalyptus Essential Oils As Obtained By Hydrodistillation Using Clevenger Type Apparatus. Biosci Biotech Res Asia 2010;7(2). Available from: https://www.biotech-asia.org/?p=8997 |
Introduction
The genus Eucalyptus includes more than 700 species and belongs to the family of Mirtacea, originally from Austria, it has spread worldwide, particularly in Africa, because of its easy adaptability and fast growth (Menut and al., 1995)
In order to improve the forest production, great efforts of reforestation based on Eucalyptus were undergone in all of Tunisia (khouja and al., 2001)
The Eucalyptus essential oil is known to be used for its therapeutic virtue. The principal active ingredient is 1-8 cineol which has strong germicidal and disinfectant properties (Lassak and Mc Carthy, 1983). Because of its antimicrobial activity, Eucalyptus essential oil may be used in food manufactory not only as a flavoring agent, but also as a conservative one (Oussalah and al., 2006).
In the present work we have firstly determinate the yield of the essential oils of 15 Eucalyptus species implanted in the Sahel of Tunisia “ Sidi Ismail Arboretum”, secondly, we have studied the chemical composition of four Eucalyptus essential oils using the gas chromatography coupled with the mass spectrometry.
The main goal of this work is to determine the difference between species, and to find the major chemical components in these Eucalyptus essential oils.
Materials and Methods
Plant Material
Samples of clean mature leaves were picked from three trees representing the same specie grown in “Sidi Ismail Arboretum” located between Msaken and Sfax, Tunisia, on January 2007. Leaves were harvested at a dry place for fifteen day. Samples used for hydrodistillation using Clevenger type apparatus were composed of leaves of fifteen different Eucalyptus species: E. torquata, E. leuxophleba, E. incressata, E. intertexta, E. transcontinentalis, E. leucoxylon, E. stricklandy, E. dumosa, E. striaticalyx, E. oleosa, E. gilii, E. salubris, E. cladocalyx, E. bicolor and E. brevifolia.
Essential oils extraction
Dried leaves of fifteen Eucalyptus species were subjected to hydro-distillation in a Clevenger-type apparatus for 3 h. The essential oils obtained were drying over anhydrous Na2SO4, and kept in a refrigerator for its further composition analysis by GC/MS.
Influence of crushing on the yield of essential oils extraction
To determine the variability of the yield of Eucalyptus essential oil extraction in accordance with crushing, leaves of E. leucoxylon were one time extremely crushed, medially crushed (10 mm of diameter), and no crushed. Each sample was then submitted to hydrodistillation (fig1).
 |
Figure 1: Diameter of leave samples.
|
Variability of the yield of essential oils in accordance with species
To investigate the influence of species on the yield of essential oil, leaves belonging to all species were medially crushed and submitted to hydrodistillation for 3 hours. To compare between species, a statistical test was carried out (SAS, version 1994).
Essential oils characterisation
Measurement of the Relative Density at 20°C (ISO 279:1998)
The relative density at 20°C is the ratio of volume of essential oil on the mass of the same volume of distilled water at 20°C. The density was measured by weighing 1 ml of essential oil in a tube, then 1 ml of distilled water.
d20 = (m2 –m0) / (m1 – m0)
m0: weigh of the tube (g)
m1: weigh of the tube + essential oil (g)
m2: weigh of the tube + distilled water (g)
Measurement of the Acid Index (ISO 1242:1999)
The acid index is the number of milligrams of Potassium hydroxide able to neutralise free acids present in one gram of essential oil. For that purpose, 2 g ± 0.005 of essential oils were weighed and diluted in 5 ml ethanol 95%. Then, 5 drops of phenolphthalein were added to the mixture, and neutralised with potassium hydroxide solution 0.1 N. Acid index was calculated:
Ia = (5.61*V)/ m
m: mass of essential oil (g)
V: Volume of the potassium hydroxide solution (ml)
Gas chromatography–mass spectrometry analysis
The GC–MS analyses were carried out using a Hewlett Packard 6890 SERIES GC system plus mass spectrometry Hewlett Packard 5973 equipped with a HP 5MS 30 m × 0.25 mm × 0.52 μm film thickness capillary column. Helium was used as carrier gas. The initial temperature of the column was 60 °C during 8 min, and then it was heated to 180 °C at a rate of 4 °C/min then held at that temperature for 10 min. Finally, it rise to 220°C at a rate of 5 °C/min and held at that temperature for 5 min. Split ratio, 1:10. 1 μl of each sample, dissolved in ethanol (1:10 v/v), was injected. The percentage composition of the oils was computed by the normalization method from the GC peak areas (Jamoussi and al., 2005). Just essential oils obtained from E. transcontinentalis, E. oleosa, E. dumosa and E. cladocalyx were analysed by GC-MS.
Identification of components
The components of the oils were identified by comparison of their mass spectra with those obtained from authentic samples and Wiley.275L mass spectral database. They were also confirmed by comparison of their retention indices (RI) and retention times (RT).
Results and Discussion
Influence of crushing on the yield of essential oil extraction
Results obtained after hydrodistillation showed that samples medially crushed (10 mm of diameter) present the highest yield of extraction (fig2). In fact, crushing is a technical practice which enlarges the contact area between solid medium (leaves) and the boiled water. In addition to that, crushing betters the extraction speed, and also the yield of hydrodistillation. However, an extreme crushing may disturb the steam circulation, and thus slowing down the extraction speed (Fellah and al., 2006).
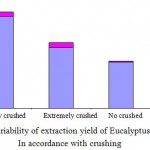 |
Figure 2: Variability of extraction yield of Eucalyptus leucoxylon In accordance with crushing
|
Variability of the yield of essential oil in accordance with species
A statistical analysis was investigated after the essential oil extraction of 15 Eucalyptus species to demonstrate that the yield of extraction depends on species (fig 3). All leave samples were medially crushed, and the hydrodisyillation was about 3 hours. Results showed that the average yield varies according to species. It is 3.63% for E. dumosa, which is the most generative of essential oil and 1.16% for E. bicolor which seems to be the less generative of essential oil.
| E13 : E. bicolor | E14: E. brevifolia | E1: E. leucoxylon | E3: E. transcontinentalis | E10: E. torquata |
| E16 : E. incressata | E5 : E. stricklandy | E11 : E. leuxophléba | E15 : E. intertexta | E8 : E. gilii |
| E12 : E. cladocalyx | E6 : E. striaticalyx | E9 : E. salubris | E4 : E. dumosa | E7 : E. oleosa |
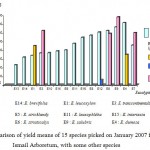 |
Figure 3: Comparison of yield means of 15 species picked on January 2007 from Sidi Ismail Arboretum, with some other species.
|
Statistical analysis using Student Newman and Keuls Test, showed the presence of six statistical group (p<0.05) (Table 1).
Table 1: SNK grouping of different yield (SAS, 1994)
| SNK grouping | Mean yield | N | Eucalyptus species |
| A
BA BC BC BC
C C
D E E E E E E E
F |
3.637
3.363 3.060 3.005 2.970
2.787
2.387 1.990 1.880 1.867 1.750 1.740 1.720 1.605
1.163 |
3
3 1 2 1
3
3 3 2 3 2 3 1 2
3 |
E. dumosa
E. salubris E. cladocalyx E. leucoxylon E. gilii
E. striaticalyx
E. leuxophleba E. stricklandy E. intertexta E. torquata E. oleosa E. incressata E. transcontinentalis E. brevifolia
E. bicolor |
N : observation number, mean yield : (g E.O/100g dry leaves)
Comparing our results to those obtained by other researchers, it was determinate that the average yield varies according species, location and season. As example, species studied by Elaissi, 2005, were E. salubris, E. transcontinentalis, E. oleosa and E. striaticalyx. These species were located in Hajeb Layoun arboretum positioned in the middle of Tunisia. Essential oils were extracted on January and June 2005.
Essential Oil Characterisation
It was noticed that E. dumosa, E. cladocalyx, E. transcontinentalis and E. oleosa belongs to three different groups concerning the yield of essential oil extraction. For that reason, only these four species were subject of the rest of this study.
Sensorial characterisation
Essential oils obtained were liquid; the colour was clear yellow for E. cladocalyx and E. dumosa, however it was dark yellow for E. oleosa and E. transcontinentalis. The odour was fresh, characteristic and persistent.
Physicochemical characteristics
Table 2: Physicochemical characteristics of essential oils.
| Eucalyptus essential oil | Relative density (20°C) | Acid index (mg KOH/ g E.O) |
| E. transcontinentalis | 0.93 | 1.40 |
| E. dumosa | 0.93 | 0.71 |
| E. oleosa | 0.92 | 3.58 |
| E. cladocalyx | 0.91 | 2.19 |
E.O.: Essential Oil
Chemical composition of four Eucalyptus essential oils
The main components of the analysed Eucalyptus oils were determinate by GC-MS analysis. Table 3 contains results of the retention index (RI) of each component, which was calculated using the followed formula:
RI = 100y + 100 [tr (x) – tr (y)/ tr(z) – tr (y)] (Dunlop and al., 1995)
tr (x) : retention time of the wanted components x
tr (y): retention time of standard n-alcane preceding the wanted component x
tr (z): retention time of the standard n-alcane following the wanted component x.
The chemical composition of essential oil is affected by several factors such as species, geographical location, harvest time, part plant used and method of isolation. In the essential oil extracted from immature leaves of four eucalyptus species: E cladocalyx, E transcontinentalis, E dumosa and E oleosa, 46 compounds were identified corresponding to more than 90% of the total oil. The four essential oils analysed consists for their major part on oxygeneted monoterpenes such as 1-8 cineole, α-campholénal. The percentages are as follows around 80%, 78%, 73% and 30% for E. transcontinentalis, E. dumosa, E. cladocalyx and E. oleosa.
Monoterpenes hydrocarbons were also present such as α thujene, α pinen and p-cymene, but in lower percentage 23% for E .oleosa, and 8% for each one of following species: E. dumosa, E. cladocalyx and E. transcontinentalis. The last group is sesquiterpenes hydrocarbons, for instance alloaromadendrene. Figures are respectively about 5%, 3.5% and 3.68% for E. dumosa, E. transcontinentalis and E. dumosa. We didn’t identify any sesquiterpenes hydrocarbons on E. cladocalyx essential oil.
 |
Figure 4 : E. dumosa chromatogram.
|
 |
Figure 5 : E. oleosa chromatogram.
|
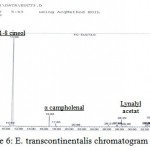 |
Figure 6: E. transcontinentalis chromatogram.
|
 |
Figure 7: E. cladocalyx chromatogram.
|
Table 3 a: Chemical composition of E. cladocalyx, E. dumosa, E. oleosa and E. transcontinentalis essential oils.
| R.I | Chemical components | %
chemical composition in E. cladocalyx |
%
chemical composition in E. dumosa |
%
chemical composition in E. oleosa |
%
chemical composition in E.transcontinentalis |
| 926 | Tricyclène | – | – | 0.66 | – |
| 931 | α thujène | 5.53 | – | 11.42 | – |
| 939 | α pinène | 2.92 | 5.72 | 0.57 | 5.71 |
| 1026 | p-cimène | – | 2.25 | 10.91 | 1.59 |
| 1033 | 1-8cinéole | 71.19 | 72.45 | 22.94 | 70.41 |
| 1040 | z-β ocimène | 0.93 | – | – | – |
| 1058 | γ-terpinène | – | 0.51 | – | – |
| 1126 | α-campholénal | 2.44 | 4.45 | 3.66 | 5.09 |
| 1139 | Trans-pinocarvéol | – | – | 0.86 | 1.32 |
| 1147 | Camphre | 0.45 | 0.79 | – | – |
| 1151 | Bornéol | – | 0.37 | – | – |
| 1154 | Trans-sabinol | – | – | 1.41 | – |
| 1163 | Pinocarvacrone | – | 0.58 | 1.58 | – |
| 1168 | Myrténal | – | 0.81 | – | – |
| 1183 | p-cimène-8-ol | – | – | – | 0.39 |
| 1217 | Trans carvéol | – | – | 0.71 | – |
| 1242 | Carvone | – | – | – | 1.23 |
| 1252 | Pipéritone | – | – | – | 0.86 |
| 1255 | Géraniol | – | – | – | 0.82 |
| 1257 | Acétate de linalyl | – | – | – | 3.27 |
| 1275 | Acétate de bornyl | – | 0.71 | – | 1.057 |
| 1298 | Carvacrole | – | – | – | 1.17 |
| 1310 | Thymol | – | – | 2.04 | – |
| 1330 | Acétate de terpényl 4 | – | 1.97 | – | – |
Table 3 b : chemical composition of E. cladocalyx, E. dumosa, E. oleosa and E. transcontinentalis essential oils
| R.I | Chemical components | %
chemical composition in E.cladocalyx |
%
chemical composition in E. dumosa |
%
chemical composition in E. oleosa |
%
chemical composition in E.transcontinentalis |
| 1350 | Acétate
D’α-terpényl |
– | 0.37 | 0.75 | – |
| 1354 | α-cubébène | – | – | 0.74 | – |
| 1371 | α-copaène | 2.49 | – | – | – |
| 1387 | β-élémène | 0.42 | – | 0.79 | – |
| 1391 | Thumbergol | – | – | 0.92 | – |
| 1394 | Longifolène | – | – | 23 | – |
| 1409 | β-cubébène | – | 0.56 | 6.03 | – |
| 1418 | β-caryophyllène | – | 0.59 | 1.7 | – |
| 1423 | Β-gurjunène | – | 3.52 | 0.85 | – |
| 1432 | α -cédrène | – | 1.17 | 1.05 | – |
| 1439 | Aromadendrène | – | 0.47 | 0.52 | – |
| 1447 | α-Himachalène | – | – | 0.63 | – |
| 1450 | Α-Humulène | – | – | 1.31 | – |
| 1460 | Alloaromadendrène | – | – | 3.48 | – |
| 1476 | γ-Murolène | – | 1.68 | 0.52 | – |
| 1532 | Elemol | 0.93 | – | – | – |
| 1542 | Α-calacorène | 0.58 | – | – | – |
| 1578 | Oxyde de β-caryophyllène | 6.74 | – | – | – |
| 1589 | Cédrol | 1.66 | – | – | – |
| 1603 | Hexadicane | 0.39 | – | – | – |
| 1625 | T cardinol | 0.37 | – | – | – |
| 1639 | α-cardinol | 0.63 | – | – | – |
| Total of identified components (%) | 97.7 | 99 | 99.1 | 93 | |
Results (table 3a, 3b) showed that there is a qualitative and quantitative difference between the four Eucalyptus essential oils tested. Some components were present in certain essential oils samples and absent in others. As example, we cite thymol, a monoterpen recognized by its antimicrobial activity (Lambert and al., 2001). This component was only ingredient of E. oleosa essential oil; it was present in 2.04%.
Myrtenal was only present in E. dumosa essential oil. The major components were α pinen, p cymen, 1-8 cineol, α campholenal, longifolen, alloaromadendren, campher, borneol and carvacrol.
Results showed that α pinen percentage was about 0.57%, 2.92% and 5.7% respectively for E. oleosa, E. cladocalyx, E. transcontinentalis and E. dumosa.
oleosa essential oils presented the most important percentage of p-cymen (10.9%), followed by E. dumosa (2.25%), then E. transcontinentalis (1.6%). This component (p-cymen), is the biological precursor of carvacrol, it is hydrophobic and causes swelling of the cytoplasmic membrane to a greater extent than does carvacrol.
P-cymen is active when combined with carvacrol (Ultee and al., 2002).
In addition to that, the study of the chemical composition showed that 1-8 cineol was the major component. The percentage was about 72.5%, 71.2%, 70.4% and 23% respectively for E. dumosa, E. cladocalyx, E. transcontinentalis and E. oleosa.
This component was abundant in all species and those results agree with (Giamakis and al., 2001) whom funded that the percentage of 1-8 cineol in Eucalyptus essential oil is about 20 till 90%.
It was concluded that the four Eucalyptus essential oils were rich in oxygenated monoterpen as carvon, α campholenal, 1-8 cineol, transpinocarvon, myrtenal, borneol, transcraveol, piperitoin and thymol. That class enclosed different active components which suppose an antimicrobial power of our essential oils (Juven and al., 1994; Dorman and Deans, 2000; Ultee and al., 2000; Lambert and al., 2001).
Conclusion
Yields of different essential oils from dried leaves of some selected Eucalyptus species varied greatly from one species to another.
46 constituents were identified in four Eucalyptus essential oils (E. dumosa, E. cladocalyx, E. transcontinentalis and E. oleosa).
1-8 cineol, α pinen, α-campholnal were prevalent constituents.
It was found that Eucalyptus oleosa essential oil possess specific active chemical components such as thymol, p-cymen recognized as antimicrobial components.
These properties of Eucalyptus essential oil tested could be used in several fields especially food alimentary as preservative.
Acknowledgements
The authors are grateful to the National Institute of research on rural genus, water and forest and to Ecole Nationale d’Ingénieurs à Gabes.
References
- Dorman, H.J.D., S.G. Deans. 2000. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. Journal of Applied Microbiology. 88: 308-316.
- Dunlop, P.J., C.M Bignell, D. B. Hibbert. 2000. Use of gas chromatograms of essential leaf oils to compare clone of Eucalyptus camaldulensis. Biochemical systematics and Ecology. 28 (4): 383-391.
- Elaissi, A. 2005. Contribution à l’étude quantitative et qualitative des huiles essentielles de 8 espèces d’Eucalyptus sp. Mastère Conservation et Valorisation des Ressources Végétales, Institut Supérieur de Biotechnologie de Monastir, Tunisie : 86 p.
- Fellah, S., M. Romdhane and M. Abderrabba. 2006. Extraction et étude des huiles essentielles de Salvia officinalis.L. cueillie dans deux régions différentes de la Tunisie. Journal de la société Algérienne de chimie. 16 (2) : 193-202.
- Giamakis, A., O. Kretsi, I. Chinou, C. G. Spyropoulos. 2001. Eucalyptus camaldulensis: volatiles from immature flowers and high production of 1,8-cineole and β-pinene by in vitro cultures. Phytochemistry. 58 (2):351-355.
- ISO 279. 1998. Huiles essentielles. Détermination de la densité relative à 20°C. Méthode de référence. 18p.
- ISO 1242. 1999. huiles essentielles. Détermination de l’indice d’acide. 18p.
- Jamoussi, B., M. Romdhane, A. M. Abderrabba, B. Ben Hassine, A. El Gadri. 2005. Effect of harvest time on the yield and composition of Tunisian myrtle oils. Flavour and Fragrance Journal. 20(3): 274-277.
- Juven, B.J., J. Kanner, F. Schved, H. Weisslowicz. 1994. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. Journal of Applied Bacteriology. 76: 626–631.
- Khouja, M.L., A. Khaldi, M.N. Rejeb. 2001. Results of the Eucalyptus introduction trials in Tunisia. Proceedings of the International conference Eucalyptus in the Mediterranean basin: perspectives and new utilization. October 15-19, 2000. Taormina. Italy: 163-168.
- Lambert, R.J.W., P. N. Skandamis, P. Coote, G.J.E .Nychas. 2001. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. Journal of Applied Microbiology. 91: 453– 462.
- Lassak, E.V. and T. Mc Carthy. 1983. Australian Medicinal Plants, North Ryde, NSW, Reeed New Holland.
- Menut, c., T. Molangui, G.E. Lamaty, J.M. Bessiere, J.B. Habibamana. 1995. Aromatic plants of tropical central Africa. 23. Chemical composition of leaf of Eucalyptus goniocalyx F. Muell and Eucalyptus patens Benth. Grown in Rwanda. J. Agric. Food. Chem. 43: 1267-1271.
- Oussalah, M., S. Caillet, L. Saucier, M. Lacroix. 2006. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157:H7, Salmonella Typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food control. 18 (5): 414-420.
- S.A.S. Statistical Analysis Software for Windows. Institute Inc. 1994. Cary, NC 27513, USA.
- Ultee, A., R. A. Slump, G. Steging, E.J. Smid. 2000. Antimicrobial activity of carvacrol toward Bacillus cereus on rice. Journal of Food Protection. 63 (5):620–624.
- Ultee, A., M. H. J Bennink, R. Moezelaar. 2002. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Applied and Environmental Microbiology. 68 (4): 1561–1568.

This work is licensed under a Creative Commons Attribution 4.0 International License.





