Manuscript accepted on : August 02, 2010
Published online on: 28-12-2010
Hana M. Gashlan and Mariam A. Mojally
Department of Biochemistry, Faculty of Science, King AbdulAziz University, Jeddah Saudi Arabia.
ABSTRACT: Diabetes mellitus is associated with derangements in the serum levels of several biochemical parameters, and type 2 Diabetes mellitus (T2DM) is a risk factor for cardiovascular diseases (CVD). The existence of hyperglycaemia enhances oxidative stress. The depletion of antioxidants (taurine) as a defensive body mechanism may augment the risk of diabetic complications. This study aims to investigate the levels of antioxidants and oxidants in T1DM, in T2DM patient and T2DM patients with cardiovascular diseases (CVD) among female Saudi diabetic patients. Our study included 130 female subjects divided into four groups, group I (T1DM), group II (T2DM), group III ( T2DM with CVD) and group IV (Control). Fasting blood samples (six ml) were collected. Glucose, glucosylated haemoglobin (HbA1c), cholesterol (C), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triacylglycerol (TAG), malondialdehyde (MDA), thiol, uric acid and nitric oxide (NO) levels were determined. The results of this study showed that taurine levels were significantly lower in all patient groups as compared to controls (p < 0.05 for all parameters). Thiol level was significantly high in GIII. Both NO and uric acid were also significantly higher in all patient groups as compared to controls. Hypercholesrterolemia, hypertriglyceridemia, high levels of LDL-c and MDA were detected in patients groups compared to control. Serum insulin level was significantly high in GII and GIII. All patients groups had significant hyperglycaemia compared to control. The female Saudi diabetic patients in this study have a higher oxidative stress status due to the high level of the production of free radicals (nitric oxide) and lipid peroxidation (malandialdehyde) and lower level of antioxidants (taurine and uric acid) in concomitant with dyslipidemia which may be due to taurine and uric acid deficiency.
KEYWORDS:
Diabetes mellitus; lipid peroxidation; oxidative stress.
Download this article as:| Copy the following to cite this article: Gashlan H. M, Mojally M. A. Blood Lipid Peroxidation (Malondialdehyde), Thiol, Taurine Levels and Oxidative Stress in Saudi Diabetic Patients. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Gashlan H. M, Mojally M. A. Blood Lipid Peroxidation (Malondialdehyde), Thiol, Taurine Levels and Oxidative Stress in Saudi Diabetic Patients. Biosci Biotech Res Asia 2010;7(2). Available from: https://www.biotech-asia.org/?p=8975 |
Introduction
Diabetes mellitus (DM) is emerging as a major public health problem in Saudi Arabia in parallel with the worldwide diabetes epidemic, which is having a particular impact upon the Middle East and the third world. DM, especially type 2 is a polygenic disorder and the high consanguinity rate among Saudis may play a significant role in its prevalence. It has been reported that Saudis have unique genetic characteristics, which may explain in part the dramatic rise in the prevalence of diabetes (El-Hazmi et al., 1995). The change in dietary habits consequently with regular consumption of large quantities of high caloric content food coupled with more sedentary lifestyle and the traditional way of life has led to high obesity. Obesity is a strong risk factor for diabetes, especially in high-risk population (Elhadd etal., 2007). Diabetes mellitus (DM) is characterized by hyperglycaemia which leads oxidative stress. DM is a risk factor for cardiovascular diseases and is also associated with derangements in oxidative status (Soliman, 2008). Depletion of body antioxidants may increase the risk of diabetic complications. Lipid peroxidation of cellular structures coupled with elevated oxygen free radicals generation, is thought to play an important role in atherosclerosis and microvascular complications of diabetes mellitus (Velazquez et al., 1991).Cellular thiols have emerged as playing an important role in the development of diabetic complications. The oxidation of the thiol groups in several peptides or proteins is specifically coupled with the reduction of other molecules. These thiol antioxidative peptides or proteins, play an important role in the cellular antioxidative defense as well as the regulation of cellular functions involving the thiol disulfide exchange (Liang and Pietrusz, 2007).
Taurine, 2-amino-ethane sulfonic acid C2H7NO3S, plays an important role in stimulating glucose mediated insulin secretion and glucose metabolism (Militante et al., 2000). In addition, taurine has been reported to protect against pathological conditions including cardiomyopathy (Redmond et al., 1998). Intracellular depletion of taurine could disturb carbohydrate metabolism and promote chronic complications of diabetes (Militante et al., 2000).
The present study aims to investigate the levels of antioxidants (taurine, uric acid and thiol) and oxidants (lipid peroxidation MDA and NO) in T1DM and in T2DM patient and T2DM patients with cardiovascular diseases (CVD) among Saudi diabetic patients.
Methods
Chemicals
Chemicals used for determination of plasma lipid peroxide (MDA) and serum thiol were purchased from Sigma-Aldrich (St Louise, MO, USA). Kits for determination of serum levels of glucose, HbA1c, uric acid, total cholesterol (TC), TAG, LDL-C and HDL-C were purchased from Dade Behring (Buckinghamshire, UK). Insulin kit was purchased from Roche Diagnostics, GmbH, Mannheium, Germany). Nitric oxide kit was purchased from (Assay Designs, Ann Arbor, Michigan, USA).
Experimental Design
A total of 100 middle class female patients (40-60 years) attending Hypertension and Diabetes Hospital in Jeddah, Saudi Arabia were involved in this study. All controls and patients were diagnosed by the hospital physicians. They were divided into three groups: Group I (GI) included 27 female patients with type I diabetes (mean age ± SE; 43.3 ± 8.3 years, duration 8.5±0.5). Group II (GII) included 28 female patients with type II diabetes (mean age ± SE; 47.3 ± 2.2 years duration 8.0±0.8). Group III (GIII) included 45 female patient with type II diabetes and suffering of Cardio Vascular Disease (CVD) which was characterized by hypercholesterolemia and hypertension (mean age ± SE; 49 ± 3, duration 9.1±0.6). Also a control group was included consisting of 30 healthy age matching female subjects (mean age ± SE; 45.6 ± 3.2). All patients and controls were not suffering of high blood pressure. Exclusion criteria for patients included cardiomyopathy, serious organ disease, systemic illness, serious psychiatric illness and anticonvulsant therapy. The NIDDM patients were not taking any medicines other than oral anti-diabetic pills.
All these groups (female patients and control subjects) were examined, diagnosed and classified by the hospital physicians. All the patients gave written, informed consent and the study was approved by the Ethics and Research Committee.
Fasting blood samples (Six ml) were collected in two tubes, one with anticoagulant added. Blood samples were used for the determination of HbA1c. Plasma samples were stored at -30° C, if not used immediately, until analysis. The plasma samples were thawed once and used for determination of taurine level using modified high-performance liquid chromatography (HPLC) (Frank and Powers, 2007), lipid peroxidation (malondialdehyde; MDA), glucose using Randox kit (Randox Laboratories, San Francisco, CA, USA). Plasma samples were subjected for determination of total thiol, insulin and nitric oxide (NO) levels. HbA1 C was determines using HPLC (John, 2003). Total TAG was determined using the Bicon kit (Bicon Diagnostik, Marienhagen, Germany). Serum TC,HDL-C and serum LDL-C were respectively determined using the bioMérieux kit (bioMérieux, Marcy l’Etoile, France).
Plasma levels of fasting blood sugar (FBS), HBA1c, cholesterol, TAG, LDL-C, HDL-C, uric acid, plasma total thiol, plasma MDA and NO were estimated. Taurine was measured by a modified method using HPLC separation of the derivatised amino acids (Frank and Powers, 2007) . It required two mobile phase, mobile phase A consisted of 30 mmol / L potassium dihydrogen phosphate buffer with 0.4 % tetrahydrofuran adjusted to pH 7.0 with 4 mol / L KOH, and mobile phase B consisted of 50 % HPLC grade acetonitrile mixed with HPLC grade water. Derivatised amino acids were prepared as follows: Three-hundred µl of plasma samples were prepared by adding 15 µl of precipitating reagent (0.5 mol /L perchloric acid). After protein precipitation, the samples were vortexed for 3 min and stored at room temperature for 10 min then centrifuged at 1200 rpm for 5 min. To 135 µl of cleared supernatant, 15 µl of internal standard (norvaline 62.5 µmol / L, Sigma) were added. The derivatised amino acids were separated on C18 column (100 mm ×4.6 mm i.d, 5 µm particle size) and C18 guard column (3.9 mm × 20 mm, 5µm particle size). The chromatographic separation was performed at room temperature (22°C) and the flow rate of the mobile phase was 1.2 ml/ min throughout the analysis. Ultra Violet detection was used (instead of fluorescent) at 334 nm.
Statistical Analysis
Statistical analysis was performed using Social Package for Social Science (SPSS) software package (SPSS Inc, Chicago, IL, USA) for data analysis version 12 for windows. The data presented in this study are expressed as mean ± SE. Student t test was used to determine the significance of the difference between groups. Data were also analysed using a one-way Analysis of Variance (ANOVA).
Results
Table I and Fig 1 show a highly significant low levels (p< 0.01) of plasma taurine level in GI and a significant low levels (p<0.05) in both GII and GIII with a percent change of -66%, -40% and -39%, respectively as compared to control. Data shown in Table I and Fig 1 reveals a significant higher level (p<0.05) of plasma thiol in T2DM with CVD patients group (GIII) as compared to control. Also, data of Table I and Fig 1 demonstrated a significant high level (p<0.0001) of NO concentration in all the studied groups compared to control. Also Table I and Fig 1 show a high level of uric acid by 22.3% in T1DM group and a higher level (51.9% and 35.6%) in T2DM and T2DM with CVD groups respectively, as compared to control.
Table 1: Plasma Levels of Taurine and Serum Levels of Thiol, NO and Uric acid in all Groups.
| Parameters
Groups |
Taurine | Thiol | NO | Uric Acid |
| umol/L
|
µmol/L × 10-4
|
µmol/L
|
mg/dl
|
|
| Control
n=30 |
||||
| Range | 41.1-66.8 | 1.6-5.8 | 4.1-11.4 | 4.1-5.9 |
| Mean±SE | 48.5±9.1 | 3.2±0.4 | 7.12±0.9 | 4.7 ± 0.3 |
| T1DM (GI)
n=27 |
||||
| Range | 29.3-34.8 | 2.2-9.0 | 10.4-17.6 | 4.2-7.8 |
| Mean±SE | 16.5±5.6 | 3.7±0.3 | 10.9±0.6 | 5.7 ± 0.2 |
| P value < | 0.01 | NS | 0.0001 | 0.05 |
| T2DM (GII)
n=28 |
||||
| Range | 17.8-33.8 | 1.4-8.0 | 9.6-17.4 | 4.30-10.10 |
| Mean±SE | 28.8±1.7 | 3.8±0.3 | 13.5±1.0 | 7.1 ± 0.3 |
| P value < | 0.05 | NS | 0.0001 | 0.001 |
| T2DM+ CVD (GIII) n=45 | ||||
| Range | 25.3-33.3 | 1.6-5.9 | 10.7-17.6 | 4.0-9.5 |
| Mean±SE | 29.4±0.9 | 3.6±0.2 | 12.1±0.6 | 6.3 ± 0.2 |
| P value < | 0.05 | 0.05 | 0.0001 | 0.001 |
SE: Standard error.
% Change : Percent change from control.
P Value < 0.05 : significant.
P Value < 0.01 : highly significant.
NS : non significant.
CVD: Cardio Vascular Disease.
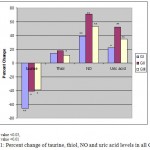 |
Figure 1: Percent change of taurine, thiol, NO and uric acid levels in all Groups.
|
Data shown in Table II and Fig 2 show an abnormal lipid profile, where TC is significantly high (P<0.05) in GI and GII and a highly significant level (P<0.001) in GIII compared to control. Serum LDL-C shows a higher levels (Table II and Fig 2) in GI, GII and GIII groups (P<0.02, 0.05 and 0.001 respectively) as compared to control. The lipid pattern also showed a highly significant elevation in TAG (in GI and GIII p<0.005 and GII p<0.05) as compared to control. In addition, serum level of MDA showed a highly significant elevation (p<0.0001) in all the studied groups compared to control.
Table 2: Serum Levels of Cholesterol, LDL, TAG, HDL and Lipid peroxide in all Groups
| Parameters
Groups |
Cholesterol | LDL | TAG | HDL | MDA |
|
mg/dl
|
mg/dl
|
mg/dl
|
mg/dl
|
µmol/µL x10-3
|
|
| Control
n=30 |
|||||
| Range | 151-176 | 100-110 | 165-179 | 30.9-45.5 | 2.11-6.9 |
| Mean ± SE | 163.3 ± 2.8 | 102.8 ± 0.9 | 171.6 ± 1.5 | 38.1 ± 1.2 | 4.5 ± 0.5 |
| T1DM (GI)
n=27 |
|||||
| Range | 128-254 | 90-183 | 115-472 | 31-57 | 6.13-13 |
| Mean ± SE | 183 ± 8.0 | 130.3 ± 8.3 | 272.1 ± 39 | 42.6 ± 1.9 | 8.3 ± 0.7 |
| P value < | 0.05 | 0.02 | 0.005 | NS | 0.0001 |
| T2DM (GII)
n=28 |
|||||
| Range | 138-250 | 91-175 | 114-400 | 31-64 | 7.98-14.9 |
| Mean ± SE | 181± 6.2 | 115.8 ± 5.0 | 201.7 ± 17.5 | 42.2 ± 1.8 | 9.6 ± 0.7 |
| P value < | 0.05 | 0.05 | 0.05 | NS | 0.0001 |
| T2DM+ CVD (GIII)
n=45 |
|||||
| Range | 122-295 | 95-179 | 157-532 | 30-89 | 7.3-13 |
| Mean ± SE | 200.6 ± 5.9 | 132 ± 4.9 | 267.2 ± 26.2 | 41.5 ± 1.7 | 8.7 ± 0.5 |
| P value < | 0.001 | 0.001 | 0.005 | NS | 0.0001 |
SE : Standard error.
% Change : Percent change from control.
P Value < 0.05 : significant.
P Value < 0.02 : highly significant.
P Value < 0.001: very highly significant.
NS : Non significant.
CVD: Cardio Vascular Disease.
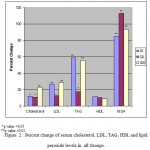 |
Figure 2: Percent change of serum cholesterol, LDL, TAG, HDL and lipid peroxide levels in all Groups.
|
As expected, fasting blood sugar and glycated hemoglobin (HbA1c) were significantly high in all the studied groups (Table III and Fig 3) as compared to control. Meanwhile, insulin serum levels were significantly higher (p<0.001) in both GII and GIII as compared to control.
Table 3: Levels of serum FBS, PPS, HbA1c, Insulin and BMI in all Groups.
| Parameters | FBS |
PPS |
HbA1c | Insulin |
| Groups | mg/dl | mg/dl | % | µU/ml |
| Control
n=30 |
||||
| Range | 75-111 | 91-130 | 5.9-9.5 | 3.99-8.3 |
| Mean ± SE | 90.9± 3.4 | 105.6±4.3 | 7.4±0.6 | 7.4± 0.6 |
| T1DM (GI)
n=27 |
||||
| Range | 80-267 | 113-365 | 6.5-12.5 | 1.7- 8.9 |
| Mean ± SE | 154.8±15.8 | 226.1±18.3 | 9.45±0.4 | 6.6 ± 0.8 |
| P value < | 0.05 | 0.001 | 0.05 | NS↓ |
| T2DM (GII)
n=28 |
||||
| Range | 78-187 | 129-416 | 6.5-13.2 | 15.7-68.9 |
| Mean ± SE | 131.1±6.9 | 220.9±16.6 | 8.8±0.3 | 28.9± 5.6 |
| P value < | 0.001 | 0.001 | 0.05 | 0.001 |
| T2DM+CVD (GIII)
n=45 |
||||
| Range | 78-297 | 90-348 | 6.7-13.8 | 15.4-52.9 |
| Mean ± SE | 168.4±9.7 | 245.9±12.1 | 9.71±0.3 | 26.9± 3.7 |
| P value < | 0.001 | 0.001 | 0.05 | 0.001 |
SE :Standard error.
% Change : Percent Change from control.
P Value < 0.05 : significant.
P Value < 0.01 : highly significant.
P Value < 0.001: very highly significant.
NS↓ : non significant decrease.
CVD: Cardio Vascular Disease.
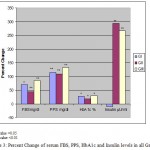 |
Figure 3: Percent Change of serum FBS, PPS, HbA1c and Insulin levels in all Groups.
|
The statistical analysis (Table IV) showed a highly significant low levels in taurine levels between control group (p<0.0125) and GI and a significant lower levels between all the studied groups (p<0.025) as compared to control.
Discussion
Most people with diabetes in developed countries by 2025 will be aged 65 years or more and this disease affecting their productive years. Around 4 million deaths worldwide every year are attributable to complications of diabetes (Al-Nozha et al., 2004). The most common secondary complications of diabetes are seen within the vascular system. Changes much like those seen with atherosclerosis in the brain and heart resulting in reduction of circulation to these areas and may result in stroke and heart attack respectively (Militante et al., 2000). Saudi Arabia is among the top 10 diabetes suffering countries. The prevalence of diabetes in Saudi Arabia, according to the epidemiological survey of Al-Nozha and colleagues was 23.7% (Al-Nozha et al., 2004).
Taurine, a free amino acid, found in high concentration in most types of animal tissues (Militante et al., 2000) and in human tissues (Odetti et al., 2003) has antioxidant properties. In the present study, plasma taurine level showed a highly significant decrease in GI and a significant decrease in both GII and GIII as compared to control. In healthy subjects taurine acts as an antiglycative compound, providing free amino groups that may compete for the reducing sugars (Odetti et al., 2003) and (Anuradha, 2009). In our study, the low in Saudi plasma taurine could be explained by higher consumption of taurine by some tissues was proposed as the cause of glucose reduced plasma levels. Other results showed a disturbed taurine content and release in platelets of T2DM patients (De Luca et al., 2001). Also, Azuma et al.(1992) suggested that diabetic patients have low level of taurine and that taurine could have beneficial effects in diabetic patients. Taurine could play a protective role as an antihypoxic (Franconi et al., 1985) and antioxidant agent (Wolff and Dean, 1987). Hansen (2001) explained the low of taurine may be due to the intracellular accumulation of glucose forming sorbitol, which is suspected to be one of the key processes in the development of diabetic late complications. Sorbitol accumulation causes depletion in intracellular osmolytes including taurine.
Lu (2009) emphasized the importance of glutathione in preventing several diseases including diabetes. The result of our study showed a significant elevation in plasma thiol levels in T2DM with CVD group as compared to control while a non significant increase was observed in T1DM and T2DM. In agreement with our results, Duman et al. (2003) declared that serum of T2DM patients had higher homocysteine (thiol) levels than control subjects. Also Ashfaq et al. (2006) stated that plasma thiols (e. g glutathione redox), a marker of oxidative stress, may be helpful in identifying individuals at risk for early atherosclerosis, independent of traditional risk factor of assessment and presence inflammation.
Nitric oxide (NO) is a small lipophilic molecule which can travel freely through cell membranes by diffusion as well as by formation of S-nitrothiols by interaction with cellular proteins. Therefore, it can act on neighboring target cells (Guo et al., 1995). Nitric oxide overproduction in diabetes has been documented in several animal models (Bank and Aynedjian, 1993) and clinical studies (Chiarelli et al., 2000). The result of our study demonstrated a highly significant elevation in NO concentration in all the studied groups compared to control. The reason for the greater NO production in patients with diabetes is unclear, but an increase in the expression of nitric oxide synthase (NOS) in these patients may play a role. Matata and Galiance (2001), found that NOx (nitrite and nitrate) concentration were higher in the diabetic patients than control. NOx was elevated in the diabetic patients with high HbA1c compared with control subjects, but nearly normal in the well controlled diabetic patients. In agreement with our results Hoeldtke et al. (2002) have found that NO over production occurs in patients with poorly controlled T1DM and leads to increased peroxy nitrite and suppressed nitric oxide. Moreover, they revealed that hyperglycaemia was the stimulus to nitrosative stress and prompted this analysis to the relationship between NO overproduction and peripheral nerve function.
Uric acid is the end product of purine catabolism in humans (Kand’ar et al., 2006) and it is also a potent non-enzymatic antioxidants (Bianchi et al., 2007). In our study, the results of uric acid showed a significant increase in T1DM group and a highly significant increase in T2DM and T2DM with CVD groups respectively, as compared to controls. Bianchi et al.(2007) observed a positive association between serum uric acid and peripheral arterial disease appeared to be independent major CVD risk factors. The mechanism by which uric acid may be associated with atherosclerotic disease remain unclear, however uric acid may play an important role in free radical formation and oxidative stress. Therefore, high serum concentrations of uric acid may be seen as an adaptive response to oxidative stress induced by hyperglycaemia. Thus, whether uric acid is a true risk factor or a powerful marker of chronic oxidative stress, remains a marker of debate (Bianchi et al., 2007).
Our data showed an abnormal lipid profile, where total cholesterol (TC) showed a significant increase in GI and GII and a highly significant increase in GIII compared to control. Hyper- cholesterolemia increases the risk of developing CVD. In our study the increase in serum TC level may be due to the decrease in taurine levels as explained by Militante and Lombardini (2000) and the overproduction of serum levels of NO causes also an elevation in cholesterol (Matata and Galiance, 2001). The results of Pinto et al. (2007) coincided with our results were they found a significant increase in cholesterol in T2DM patients.
Also, Elvevllo et al. (2008) suggested that the anti-hypercholesterol effects of taurine may be due to its ability to promote bile salt formation which in turn conjugates with cholesterol derivates to form taurocholate. This is considered the major bile salt that extracts cholesterol from plasma in humans. Meanwhile, diminished taurine content is associated with a high blood cholesterol and increased risk of atherosclerosis. The anti-atherosclerotic effects of taurine have been studied in different hypercholesterolaemic and hyperlipidemic animals, but the exact mechanism of action is still unclear. It has been found that, the decrease in the cholesterol level in the serum by taurine is mainly due to the decrease in LDL. Taurine lowers cholesterol by repressing TAG secretion from the liver (Chen et al., 2004).
The results of our study showed a significant increase in LDL-C serum levels in GI, GII and GIII groups as compared to control. LDL-C is a well-established risk factor for susceptibility to CVD (Chen et al., 2004). It has been documented that high levels of cholesterol and LDL-C play a significant role in the development of arteriosclerosis and hence coronary heart disease, and as shown by the results of El-hazmi et al. (1999) Saudi T2DM patients with a higher prevalence of lipid abnormalities (dyslipidemia) constitute a moderate to high risk group for the development of coronary heart disease. Alizadeh et al. (2008) stated that patients with T2DM are known to suffer from high rates of cardiovascular disease. Hossein et al. (2007) declared that diabetic patients are under high oxidative stress where hyperglycaemia plays an important role in LDL-C oxidation. Levels of acute and chronic hyperglycaemia correlate strongly with measures of LDL-C oxidation. It has been suggested that the oxidative modification of LDL-C could promote and accelerate the development of atherosclerosis. Bianchi et al. (2007) highlighted that smaller LDL-C particle size is associated with increased intima-medithickness and with the presence of coronary heart disease in T2DM.
In our study, the lipid pattern also showed a highly significant enhancement in TAG in GI and GIII and a non significant increase in HDL-C serum levels in all the studied groups as compared to control. These results may not show a typical dyslipidemic lipid profile but it showed a disturbance in lipid profile as a result of T2DM. The mechanisms responsible for hypertriglyceridemia may be an increased hepatic secretion of VLDL and a delayed clearance of TAG-rich lipoproteins, which might mainly be due to increased levels of substrates for TAG production, free fatty acids, and glucose. Hypertriglyceridemia usually accompanies decreased HDL-C, which is also a prominent feature of plasma lipid abnormalities seen in diabetic subjects (Howard, 1987) and (Yoshioka et al., 1979). Other study performed by Valdivielso et al.(2007) stated that, diabetic dyslipidemia, characterized by a fasting profile of high TAG levels, low HDL-C concentrations contributes to a greater incidence of arteriosclerotic disease in T2DM. The low level of HDL-C, which exerts anti-atherogenic and antioxidative effects when present in sufficient amounts, is a key feature of T2DM. The reduced HDL-C levels, often accompanied by elevations in plasma TAG levels (Lamarche et al., 1996) exerts anti-atherogenic and antioxidative effects when present in sufficient amounts, is a key feature of T2DM. Taurine plays an important role in lipid metabolism such as decreasing cholesterol by the enhancement of bile acid formation, and decreasing triacylglycerol which might improve lipid metabolism and insulin resistance in T2DM in rat model (Nakaya et al., 2000).
On the other hand, Warren (2007) a researcher at the University of Kentucky suggested that, at least for people with diabetes, having high levels of HDL-C in the blood stream there might be an increase risk for cardio vascular disease. He explained, in people with diabetes, HDL-C molecules bind with a natural compound called myristic acid, which somehow causes the HDL-C to inhibit the body’s natural production of NO, a substance known to protect against cardiovascular disease.
Lipid peroxidation is a free radical related process which is potentially harmful because it is uncontrolled, self enhancing process causes disruption of membranes, lipids and other cell components. In our present study, serum level of MDA showed a highly significant increase in all the studied groups compared to control. It has been found to be connected with various disease processes, such as atherosclerosis and hypertension (Mahboob et al., 2005). Duman et al. (2003) and Hossein et al.(2007), results coincided with our findings and they stated that plasma MDA levels were significantly increased in T2DM. Mahboob et al.(2005) revealed that the high levels of MDA clearly show that diabetic patients, irrespective of the gender, were exposed to an increased oxidative stress via lipid peroxidation. Harris (1992) found that a decreased level of glutathione (thiol) indicates reduced scavenging capacity of glutathione-dependent anti-oxidant defensive system against elevated lipid peroxidation processes in T2 DM patients.
Therefore, our previous results of plasma taurine level as well as serum thiol, NO, uric acid, abnormal lipids profile (dyslipidemia) and extensive lipid peroxidation showed that the Saudi diabetic and diabetic with CVD patients are under oxidative stress.
In our study, as expected, a significant high level of fasting blood sugar and PBS, glycated hemoglobin (HbA1c) were found in all the studied groups as compared to control. Meanwhile insulin serum levels showed a highly significant increase in both GII and GIII and a non significant decrease in GI as compared to control. Insulin plasma levels were low in patients of T1DM patients of G1 could be explained as they are insulin dependent and traces of insulin might be still in their blood. On the other hand, Shanik et al.(2008) revealed that hyperinsulinemia is often both a result and driver of insulin resistance. They recognized insulin resistance as a strong predictor of disease in adults which has become the leading element of the metabolic syndrome and renewed as a focus of research. The condition exists when insulin levels are higher than expected relative to the level of glucose. Thus insulin resistance is by definition tethered to hyperinsulinemia (Rosenson, 2005). Resistance to insulin likely underlies the changes that occur in lipid parameters of T2 DM, and usually it is associated with higher concentrations of cholesterol and TAG, and lower concentrations of HDL-C (Garvey et al., 2003).
In the present study, the relationship of taurine level between all the studied groups and control showed a significant decrease in taurine levels in all diabetic patients as compared to their respective controls. On the other hand, a non significant change in taurine level was detected between T2DM and T2DM+CVD patients. In the view of the results of Winiarska et al.(2009) he concluded that taurine-induced increase in the activities of catalase and the enzymes of glutathione metabolism is of importance for antioxidative action of this amino acid and thus, taurine seems to be beneficial for the therapy of diabetes.
In conclusion, these results suggest that oxidative stress in Saudi female diabetic patients can be attributed to an increase in the production of free radicals (nitric oxide) and increased lipid peroxidation (MDA) and the decrease in antioxidants (taurine and uric acid) in concomitant with dyslipidemia which may be due to taurine and uric acid deficiency. So, our results suggest that there seems to be an imbalance between plasma oxidant and antioxidant systems in patients with NIDDM. Our results also suggest the possibility of a therapeutic protocol of giving taurine and other antioxidants as food supplements to Saudi patients, hoping that this may improve the obvious dyslipidemia, oxidative stress and decrease suffering from diabetes complications specially CVD.
Acknowledgement
The authors wish to express their sincere appreciation to Dr. Naglaa Sherif associated professor in Biochemistry Department, KAU for her assistance and support.
References
- El-Hazmi MA, al-Swailem AR, Warsy AS, et al. Consanguinity among the Saudi Arabian population. J Med Genet 1995; 32: 623-6
- Alizadeh Dehnavi R, Beishuizen ED, van de Ree MA, et al. The impact of metabolic syndrome and CRP on vascular phenotype in type 2 diabetes mellitus. Eur J Intern Med 2008; 19: 115-21
- Al-Nozha MM, Al-Maatouq MA, Al-Mazrou YY, et al. Diabetes mellitus in Saudi Arabia. Saudi Med J 2004; 25: 1603-10
- Anuradha CV. Aminoacid support in the prevention of diabetes and diabetic complications. Curr Protein Pept Sci 2009; 10: 8-17
- Ashfaq S, Abramson JL, Jones DP, et al. The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults. J Am Coll Cardiol 2006; 47: 1005-11
- Azuma J, Sawamura A, Awata N. Usefulness of taurine in chronic congestive heart failure and its prospective application. Jpn Circ J 1992; 56: 95-9
- Bank N, Aynedjian HS. Role of EDRF (nitric oxide) in diabetic renal hyperfiltration. Kidney Int 1993; 43: 1306-12
- Bianchi C, Penno G, Pancani F, et al. Non-traditional cardiovascular risk factors contribute to peripheral arterial disease in patients with type 2 diabetes. Diabetes Res Clin Pract 2007; 78: 246-53
- Chen W, Matuda K, Nishimura N, Yokogoshi H. The effect of taurine on cholesterol degradation in mice fed a high-cholesterol diet. Life Sci 2004; 74: 1889-98
- Chiarelli F, Cipollone F, Romano F, et al. Increased circulating nitric oxide in young patients with type 1 diabetes and persistent microalbuminuria: relation to glomerular hyperfiltration. Diabetes 2000; 49: 1258-63
- De Luca G, Calpona PR, Caponetti A, et al. Taurine and osmoregulation: platelet taurine content, uptake, and release in type 2 diabetic patients. Metabolism 2001; 50: 60-4
- Duman BS, Ozturk M, Yilmazeri S, Hatemi H. Thiols, malonaldehyde and total antioxidant status in the Turkish patients with type 2 diabetes mellitus. Tohoku J Exp Med 2003; 201: 147-55
- Elhadd TA, Al-Amoudi AA, Alzahrani AS. Epidemiology, clinical and complications profile of diabetes in Saudi Arabia: a review. Ann Saudi Med 2007; 27: 241-50
- El-Hazmi MA, Al-Swailem AR, Warsy AS, et al. Lipids and related parameters in Saudi type II diabetes mellitus patients. Ann Saudi Med 1999; 19: 304-7
- Elvevoll EO, Eilertsen KE, Brox J, et al. Seafood diets: hypolipidemic and antiatherogenic effects of taurine and n-3 fatty acids. Atherosclerosis 2008; 200: 396-402
- Franconi F, Stendardi I, Failli P, et al. The protective effects of taurine on hypoxia (performed in the absence of glucose) and on reoxygenation (in the presence of glucose) in guinea-pig heart. Biochem Pharmacol 1985; 34: 2611-5
- Frank MP, Powers RW. Simple and rapid quantitative high-performance liquid chromatographic analysis of plasma amino acids. J Chromatogr B Analyt Technol Biomed Life Sci 2007; 852: 646-9
- Garvey WT, Kwon S, Zheng D, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes 2003; 52: 453-62
- Guo FH, De Raeve HR, Rice TW, et al. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci U S A 1995; 92: 7809-13
- Hansen SH. The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev 2001; 17: 330-46
- Harris ED. Regulation of antioxidant enzymes. FASEB J 1992; 6: 2675-83
- Hoeldtke RD, Bryner KD, McNeill DR, et al. Nitrosative stress, uric Acid, and peripheral nerve function in early type 1 diabetes. Diabetes 2002; 51: 2817-25
- Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world–a growing challenge. N Engl J Med 2007; 356: 213-5
- Howard BV. Lipoprotein metabolism in diabetes mellitus. J Lipid Res 1987; 28: 613-28
- John WG. Haemoglobin A1c: analysis and standardisation. Clin Chem Lab Med 2003; 41: 1199-212
- Kand’ar R, Zakova P, Muzakova V. Monitoring of antioxidant properties of uric acid in humans for a consideration measuring of levels of allantoin in plasma by liquid chromatography. Clin Chim Acta 2006; 365: 249-56
- Lamarche B, Despres JP, Moorjani S, et al. Triglycerides and HDL-cholesterol as risk factors for ischemic heart disease. Results from the Quebec cardiovascular study. Atherosclerosis 1996; 119: 235-45
- Liang M, Pietrusz JL. Thiol-related genes in diabetic complications: a novel protective role for endogenous thioredoxin 2. Arterioscler Thromb Vasc Biol 2007; 27: 77-83
- Lu SC. Regulation of glutathione synthesis. Mol Aspects Med 2009; 30: 42-59
- Mahboob M, Rahman MF, Grover P. Serum lipid peroxidation and antioxidant enzyme levels in male and female diabetic patients. Singapore Med J 2005; 46: 322-4
- Matata BM, Galinanes M. Effect of diabetes on nitric oxide metabolism during cardiac surgery. Diabetes 2001; 50: 2603-10
- Militante JD, Lombardini JB, Schaffer SW. The role of taurine in the pathogenesis of the cardiomyopathy of insulin-dependent diabetes mellitus. Cardiovasc Res 2000; 46: 393-402
- Nakaya Y, Minami A, Harada N, et al. Taurine improves insulin sensitivity in the Otsuka Long-Evans Tokushima Fatty rat, a model of spontaneous type 2 diabetes. Am J Clin Nutr 2000; 71: 54-8
- Odetti P, Pesce C, Traverso N, et al. Comparative trial of N-acetyl-cysteine, taurine, and oxerutin on skin and kidney damage in long-term experimental diabetes. Diabetes 2003; 52: 499-505
- Pinto X, Corbella E, Figueras R, et al. [Factors predictive of cardiovascular disease in patients with type-2 diabetes and hypercholesterolemia. ESODIAH study]. Rev Esp Cardiol 2007; 60: 251-8
- Redmond HP, Stapleton PP, Neary P, Bouchier-Hayes D. Immunonutrition: the role of taurine. Nutrition 1998; 14: 599-604
- Rosenson RS. HDL-C and the diabetic patient: target for therapeutic intervention? Diabetes Res Clin Pract 2005; 68 Suppl 2: S36-42
- Shanik MH, Xu Y, Skrha J, et al. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care 2008; 31 Suppl 2: S262-8
- Soliman GZ. Blood lipid peroxidation (superoxide dismutase, malondialdehyde, glutathione) levels in Egyptian type 2 diabetic patients. Singapore Med J 2008; 49: 129-36
- Valdivielso P, Hidalgo A, Rioja J, et al. Smoking and postprandial triglycerides are associated with vascular disease in patients with type 2 diabetes. Atherosclerosis 2007; 194: 391-6
- Velazquez E, Winocour PH, Kesteven P, Alberti KG, Laker MF. Relation of lipid peroxides to macrovascular disease in type 2 diabetes. Diabet Med 1991; 8: 752-8
- Warren J. The future of coronary heart disease prevention. Clin Med 2007; 7: 524-5; author reply 5-6
- Winiarska K, Szymanski K, Gorniak P, Dudziak M, Bryla J. Hypoglycaemic, antioxidative and nephroprotective effects of taurine in alloxan diabetic rabbits. Biochimie 2009; 91: 261-70
- Wolff SP, Dean RT. Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem J 1987; 245: 243-50
- Yoshioka T, Kawada K, Shimada T, Mori M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am J Obstet Gynecol 1979; 135: 372-6.

This work is licensed under a Creative Commons Attribution 4.0 International License.





