Manuscript accepted on : September 08, 2010
Published online on: 28-12-2010
Aerobic Hexavalent Chromium Reduction by Acenetobacter Calcoaciticus
V. Mishra, D. P. Samantaray*, S. K. Dash, A. K. Sethi and B. B. Mishra
Post Graduate Department of Microbiology, Centre for Post Graduate Studies Orissa University of Agriculture and Technology, Bhubaneswar - 751 003 India.
Corresponding Author E-mail:dpsamantaray@yahoo.com
ABSTRACT: Rapid industrialization and development of chemical and mining industries in Sukinda mining area, district of Jajpur, Orissa has drawn worldwide attention as one of the most polluted area with hexavalent chromium due to chromite rich. It is pertinent mention that hexavalent chromium is highly toxic to living beings including the flora & fauna around Sukinda mining area. On account of that, an attempt has been made in the present investigation to detoxify Cr(VI) by employing a lyophilized Gram-negative bacteria i.e. A. calcoaciticus isolated from that area. It was observed that A. calcoaceticus tolerated 1000ppm of hexavalent chromium. It shown 78.03% hexavalent chromium reduction when incubated in a nutritive medium at 30°C for 24 hours at pH 7 and the rate of reduction decreased from 78.03 to 38.1% in a nonnutritive medium. Then the bacteria was selected for parametric studies and observed to exhibit highest reduction 85% at pH 8.0, temperature 30/ 24 hours. Therefore, A. calcoaceticus may be used for bioremediation of hexavalent chromium toxicity in different chromium contaminated sites.
KEYWORDS: Bioremediation; Hexavalent chromium; lyophilized; Nutritive medium
Download this article as:| Copy the following to cite this article: Mishra V, Samantaray D. P, Dash S. K, Sethi A. K, Mishra B.B. Aerobic Hexavalent Chromium Reduction by Acenetobacter Calcoaciticus. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Mishra V, Samantaray D. P, Dash S. K, Sethi A. K, Mishra B.B. Aerobic Hexavalent Chromium Reduction by Acenetobacter Calcoaciticus. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9588 |
Introduction
Extensive utilization of minerals for human need finds application in various industries. Therefore, mining activities along with rapid industrialization is generally considered as index of progress in any country. Our country is endowed with various types of minerals. Sukinda valley of Jajpur district, Orissa the fourth most polluted place in the world1 accounts for 97% of India’s chromites ore deposits and last 70 years of intensive open cast mining generated 30 million tones of hexavalent chromium wastes polluting soil, water, ruined agricultural fields and slowly poisoned 2.6 million people of Orissa. The environment is sensitive to metals due to their longevity and toxicity2. Chromium an essential micronutrient in animal physiology required for normal carbohydrate and lipid metabolism3 and also a priority pollutant is well known for the mutagenicity4, carcinogenicity5 and teratogenicity6 of its hexavalent form in humans, experimental animals7 and Plants8. A common water pollutant, the Cr(VI) form is toxic to most organisms because of its strong oxidizing properties. It is the water soluble, bioleachable form that can intracellularly reduced to Cr(V) and reacts with nucleic acids and other cell components to produce mutagenic and carcinogenic effects on biological systems9. Conventional chemical treatment of Cr(VI) waste generating large volume of sludge, dangerous gases and expensive cost of the chemical reducing agents makes it imperative to look into safer and cheaper alternatives, where the metal resistant microorganisms are of primary importance in bioremediation of metal contaminated sites. Bioreduction of Cr(VI) occurs directly due to microbial metabolism or indirectly by bacterial metabolites10. Many researcher11, 2, 12 have investigated and demonstrated the feasibility for treatment of Cr(VI) contaminated sites and industrial effluents using either pure culture or consortium of bacteria. Bacterial Cr(VI) reduction by locally isolated strains and optimization of factors involved in Cr (VI) reduction by monoculture of isolated bacteria was carried out to investigate its efficiency as an efficient tool for bioremediation of chromium contaminated sites.
Materials and Methods
Culture collection
A lyophilized Gram-negative bacteria culture of A. calcoaciticus was taken for hexavalent chromium reduction study. This bacterium was isolated from Sukinda mining area of Jajpur district, Orissa. The culture was revived by inoculated into the broth, incubated at temperature 30 overnight and were streaked onto Luria Bertani agar (LA) plates, which were further incubated for 24 hours. Luria Bertani broth and agar were used for bacterial culture revival and chromium reduction studies procured from HiMedia Laboratories, Mumbai.
Estimation of heavy tolerance
For estimation of chromium tolerance molten LA medium was supplemented with Cr(VI) with final concentration ranging from 100-1100mg/l by using filter (0.45µm) sterilized K2Cr2O7 solution. The isolate was streaked onto the LA plates and incubated at temperature 30 for 48 hours and the resistance pattern or minimum inhibitory concentration (MIC) was noted down. Then the isolate was selected for further reduction studies basing on their chromium tolerance. Similarly, other metal tolerance study was also carried by varying the concentration (100-1000ppm) of each heavy metal such as Iron, Copper, Nickel, Mercury and Cobalt.
Estimation of pH tolerance
The pH tolerance test was conducted to study the cardinal pH of the chromium resistant bacteria. Five milliliter of the medium was taken in different test tubes and the pH was adjusted from 3-12 with help of 1N
HCL, 1N NaOH. 100µl of the overnight culture (LB) was dispensed in to the test tubes and incubated at temperature 30 for 24hours. A loopful of overnight culture was subcultured on to LA plates and all the plates were incubated at temperature 30 for 24 hours. Then the cardinal pH was determined from the observation.
Aerobic hexavalent chromium reduction
For this study, A. calcoaciticus was inoculated into LB with 100ppm of Cr (VI) and incubated at temperature 30 for 24 hours/ 100rpm with incubator shaker (STM-225-IS). Cells were collected after centrifugation (Remi Compufuge) at 10,000rpm for 10 minutes and the supernatant was analyzed for residual chromium by 1, 5-Diphenyl carbazide method13. In order to observe chromium reduction in a non-nutritive medium like Phosphate buffer solution (PBS) the collected cells were washed twice with PBS and resuspended in 50ml of PBS with 100ppm of Cr(VI) and reduction was observed at hourly intervals up to 6 hours and finally after 24 hours. Cr (VI) reduction was determined by measuring absorbance at 540nm using a spectrophotometer (Systronic 104).
Optimization of pH and temperature on chromium reduction
The influences of pH and temperature on chromium reduction were assessed with LB medium and culture condition described earlier for chromium reduction. For the effect of pH, autoclaved culture medium was adjusted to pH 7 and 8 with HCL or NaOH and incubated at temperature 30. Similarly keeping the optimum pH constant for reduction, temperature was varied viz., 20, 30 and 37 and optimum temperature was observed for chromium reduction.
Results and Discussion
Heavy metal tolerance profile
It was observed from the study that, A. calcoaceticus could tolerate Cr(VI) 1000ppm. Similar results have been found by most of the researcher12, 14, 15 while working on Cr(VI) chromium reduction. Natural habitats are generally characterized by the co-existence of a large number of toxic and non-toxic cations and therefore, it is necessary to study multiple metal effects on the physiology and biochemistry of microorganisms16. A. calcoaceticus showed a broad range of tolerance to heavy metals such as Iron, Copper, Nickel, Mercury and Cobalt up to concentration of 1000ppm, 900ppm, 1000ppm, 100ppm and 300ppm respectively. Apart from all the metals, the highest tolerance was observed towards Iron and Nickel .These observations assume great significance11 because effluents from any metal related to industry or mines have several metal ions or contaminants. Tolerance to other metals has an added advantage of withstanding the presence of other metal ions while performing the desired activity.
pH tolerance profile
The pH tolerance profile results reveal that the A. calcoaceticus grows well in between 7-9 pH and alkaline pH favors the growth of the isolate than acidic. This result corresponds to that of 11, 14 , who observed that isolates more tolerant to Cr(VI) grew better at pH 7-9. This might be a result of adaptation of the isolate to the natural habitat which was mostly alkaline.
Aerobic hexavalent chromium reduction
Hexavalent chromium reduction is dependent upon pH, temperature and Cr(VI) concentration. The results of hourly hexavalent chromium reduction presented Graph1 in a nutritive media (LB) reveals A. calcoaceticus reduced Cr(VI) by 76.01% at temperature 30/24 hours/pH 7. The trend increases with increase in time i.e. up to twenty-fourth hours. The increase in reduction may be due to increase in cell mass and cell density. But the rate of reduction was decreased from 76.01% to 38.1% in a non-nutritive medium (PBS). This difference in trend of reduction in a non-nutritive medium may be due to decrease in physiological and metabolic activities of the isolates11, 17, 14, 10 and viability after some time and possible inhibition of biomass activity by prolonged chromate toxicity in a non-nutritive medium.
Optimization of pH on chromium reduction
An hourly chromium reduction study in LB presented in Graph2 indicates that, A. calcoaceticus reduced 85% hexavalent chromium optimum at pH 8. The trend increases with increase in time i.e upto twenty fourth hour. The increase in reduction may be due to increase in cell mass and cell density. Similar results were also obtained by18, reporting that Cr(VI) reduction in Enterobacter cloacae occurred at pH 6.5-8.5 and was inhibited at pH 5-9. As Cr(VI) reduction is enzyme mediated, changes in pH will affect the degree of ionization of the enzyme changing the protein conformation and affecting the enzyme activity19.
Optimization of temperature on chromium reduction
A similarly hourly study of hexavalent chromium reduction by the organism in LB presented in Graph3 indicates that percentage of reduction (85%) is higher at optimum temperature of 30°C. An increasing trend of reduction was also observed with increase in time due to inoculation of more number of cells. Several different researchers11, 14, 10 also reported an optimum temperature of 30-37 for chromate reduction. But18 reported that no chromate reduction was observed at temperature 4 and 60. Temperature is an important selection factor for bacterial growth and affects enzymatic reactions necessary for chromate reduction.
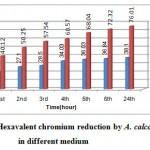 |
Graph 1: Hexavalent chromium reduction by A. calcoaceticus in different medium.
|
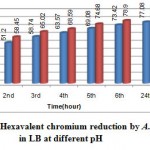 |
Graph 1: Hexavalent chromium reduction by A. calcoaceticus in different medium.
|
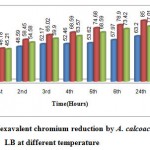 |
Graph 3: Hexavalent chromium reduction by A. calcoaceticus in LB at different temperature.
|
Conclusion
Hence, the present investigation has examined the presence of indigenous bacteria from the Cr (VI) contaminated sites of sukinda mining area. The experimental observations indicate that the locally isolated strain show high Cr(VI) tolerance and demonstrate good metal removal capability. The advantages of selecting the indigenous bacteria from chromite mines of Sukinda for bioremedial purpose may be the minimization of inhibitory effects from other components that may be present along with Chromium, since viable indigenous bacterial isolates will have developed some degree of resistance to these components.It might be practical to use Cr(VI) reducing bacteria to reduce other waste metals simultaneously, which shows a positive sign for application of these locally isolated strains in the treatment or detoxification of hexavalent chromium from industrial effluents and Chromium contaminated sites. But before exploiting the strain as an efficient biotechnological tool for chromium detoxification further investigation needs to be carried out in laboratory scale and in-situ metal reduction potential of the Genus has to be assessed.
Acknowledgements
I would like to acknowledge Orissa Mining Corporation and State pollution control board for giving the permission to conduct the study in Sukinda mining area. I also like to thank laboratory staff of department of Biochemistry, Anatomy and Animal nutrition, College of Veterinary Science and Animal Husbandry, Orissa University of Agriculture and Technology, Bhubaneswar, Orissa for their co-operation.
References
- Blacksmith Institute Report, 16-17 (2007).
- Aravindhan R., Sreeram K.J., Rao J. R. and Nair B.U., The Journal of General and Applied Microbiology, 53(2), 71-79 (2007).
- Anderson R. A., Sci. Tot. Environ., 86, 75-81 (1989).
- Petrilli F. L. and Flora S. D., Appl. Environ. Microbiol., 33, 805–809 (1977).
- Gruber J. E. and Jennette K. W., Biochem. Biophys.Res. Commun., 82, 700–706 (1978).
- Gale T. F., Environ. Res., 16, 101–109 (1978).
- IARC, International Agency for Research on Cancer, Lyon, France, Vol. 49, 677 (1990).
- Flora S. D., Bagnasco M., Serra D., and Zanacchi P., Mutat. Res., 238, 99–172 (1990).
- McLean J. and Beveridge T. J., Applied and Environmental Microbiology, 67(3), 1076-1084 (2001).
- Losi M.E., Amrhein C. and Frankenberger W.T., Rev. Environ. Contam. Toxicol., 36, 91–121 (1994a).
- Das A. K., Mishra S. and Jena S. Master of technology (research) in Chemical engineering thesis, Department of chemical engineering, National institute of technology, Rourkela, Orissa, India, 26-56 (2009).
- Rahman M.U., Gul S., Haq Z.U., Turk. J. Biol ., 311, 61-66 (2007).
- APHA, 18th American Public Health Association, Washington, D.C., 981 (1992).
- Camargo F.A., Bento F.M.,Okeke B.C and Frankenberger W.T., J. Environ. Qual ., 32, 1228-1233 (2003).
- Losi M.E. and Frankenberger W.T., Water Air Soil Pollut., 74, 405–413 (1994).
- Verma S. K. and Singh S. P., Bull. Environ. Contam. Toxicol. 54, 614-619 (1995).
- Zakaria Z. A., Surif S. and Ahmad W. A., Journal of Hazardous Materials, 146(1-2), 30-38 (2007).
- Wang P., Mori T., Toda K. and Ohtake H., J. Bacteriol., 172, 1670–1672 (1990).
- Farrell S.O. and Ranallo R.T., Experiments in biochemistry-A hands-on approach, Saunders College Publ., Orlando, FL. (2000).

This work is licensed under a Creative Commons Attribution 4.0 International License.





