Manuscript accepted on : July 21, 2010
Published online on: 28-12-2010
A Study on Poly Hydroxy Butyrate (PHB) Production and Its Application from Pseudomonas Spp. (MTCC)
S. Sujitha1 and D. Ravi2
1CMS College of Science and Commerce, Coimbatore - 641 006 India.
2Government Arts College, Higher Education Department, Coimbatore - 641 018 India.
ABSTRACT: By considering the industrial interest on poly hydroxyl butyrate and its high production cost current work has been undertaken for the production of PHB from five different Pseudomonas species. By using different types of substrate waste, like Food Industry wastes(Bagasse,Wheat bran, Whey), Chemical Industrial waste (Activated sludge) and Wood industry waste (saw dust ) were used as a cheap substrate to minimize the production cost of PHB.Accumulate through metabolic mechanism microbes accumulate secondary metabolites such as PHB in the form of granules in the intracellular region. This was analyzed and identified by Nile blue A and Sudan black method.PHB production in various industrial waste based medium was studied by Crotonic acid method. The pure form of PHB was collected and biochemically analyzed by TLC method .The biodegradation of PHB was studied by using Fungal isolates through PHB incorporated agar medium assay. The clear zone around the colonies was measured to evaluate the activity of the isolates. The fungal isolates were found to degrade these polymers more rapidly within 3 to 7 days due to their versatile depolymerase activities. These PHB has another role in biochemical mechanisms.ie, poly 3 hydroxy butyrate act against the pathogens in the fishes and other aquaculture spp.(specifically in nauplii-var.).
KEYWORDS: Poly- b- hydroxy butyrate; PHB; Bioplastic; Biodegradation fungi; PHB application.
Download this article as:| Copy the following to cite this article: Sujitha S, Ravi D. A Study on Poly Hydroxy Butyrate (PHB) Production and Its Application from Pseudomonas Spp. (MTCC). Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Sujitha S, Ravi D. A Study on Poly Hydroxy Butyrate (PHB) Production and Its Application from Pseudomonas Spp. (MTCC). Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9375 |
Introduction
Almost every product we buy most of the food we eat and many of the liquids we drink come encased in plastic packaging, which provides excellent protection for the product. It is cheap to manufacture and seems to last forever. Lasting forever, however, is proving to be a major environmental problem. Plastics are manufactured from numerous non-renewable resources like natural gas, coal, and oil. Plastics cannot be degraded (xenobiotic compounds) easily in the natural environment due to their long polymer molecules which are too large and too tightly bound.
The following are few items and the time required for their decomposition, Tin cans- 50 to 100 years., Aluminum Cans- 80 to 100 years., Glass Bottles- 1 million years., Newspapers- 25 to 50 years., Polystyrene- 1000’s of years., and Plastic bags- 400 years
A type of study on hard plastic is made with a molecule known as Bisphenol A, BPA. BPA like many other man made chemicals is now detectable in most people’s blood streams and could cause dangerous hormonal changes in children. BPA may tend to cause cancer, early puberty, obesity and even attention deficit disorder.
Every year several thousand tones of plastic wastes are discarded into marine environment and accumulate in certain oceanic regions. Approximately , one million marine animals are killed every year either by chocking of plastics as they mistake it for food or become entangles in non- degradable plastic debris (Fiechler,1990).The synthesis of some polymeric substances involves the use of toxic compounds or the generation of toxic by-products ,which is another problem with these non degradable plastics . These problems with these non degradable plastics. These problems have increased the attention on polymers that are derived from biological precursors or they are produced by using modern biotechnological Methods, which are biodegradable .The polymers thus obtained are known as biopolymers.
To overcome this problem Biodegradable Plastics have been developed which are made from renewable resources, such as plants. The plastics that are decomposed in the natural environment are known as the “biodegradable plastics”. (‘Biodegradable’ means that a substance can naturally decompose with the help of micro organisms and will not persist in the environment beyond a certain period of time). The chemical bonds of biodegradable compounds are easily destroyed by a variety of bacteria over a small period of time to facilitate their decomposition. The most widely studied bioplastic is poly hydroxyl butyrate.
As early as in the year 1926, Lemoigne managed to isolate the first of the polyhydroxyalkanoates – polyhydroxybutyrate (a homopolymer whose building unit is the 3-hydroxybutyric acid) from the Bacillus megaterium bacterium. At the end of the 1950s, the presence of the polyhydroxybutyrate was confirmed as an energy and carbon source and storage in many other bacteria. Many species of bacteria accumulate polyhydroxyalkonoates as energy storage compounds, some of the PHA polymers are commercially valuable as biodegradable plastics.
PHB has been shown to be biodegradable by bacteria into water and carbon dioxide (and methane under anaerobic conditions) in natural environments including water, soil and compost. Fungi play a considerable role in degrading polyesters, just as they predominantly perform the decomposition of organic matter in the soil ecosystem (Kim and Rhee, 2003). The fungal isolates were found to degrade these polymers more rapidly when compared to bacteria, due to their versatile depolymerase activities.
Vibriosis is one of the major disease problems in aquaculture and is a bacterial disease responsible for mortality of cultured shrimp worldwide (Lightner & Lewis, 1975; Adams, 1991; Chen et al., 2000). Vibriosis is ubiquitous throughout the world. vibrio spp occur naturally in aquatic environments. Infections caused by luminescent vibrios can cause dramatic losses in aquaculture. These infections are often hard to treat with antibiotics because of the spread of resistant strains and therefore, alternative strategies were used. The short-chain fatty acid of poly hydroxybutyrate protects most fish from Vibrio infection.
Though a number of standard and patented starins are available in the research laboratories, the present investigation was carried out to identify PHA and PHB accumulating microbes and its production using less expensive substrates with the following objectives.
Identifying the microbes, utilizing various wastes as a substrate.
Screen the PHB accumulating Pseudomonas species by phenotypic methods.
Identify the maximum PHB accumulating MTCC Pseudomonas species by cell distruption methods.
Estimate PHB by varying intrinsic and extrinsic factors.
Extraction and analysis of accumulated PHB by biochemical method such as TLC.
Study the bio degradation of biopolymer by using a fungus Aspergillus species.
Role of PHB in controlling pathogenic diseases in aquaculture organisms.(specifically in nauplii-var.)
Materials and Method
Organism
Pseudomonas sp (P.aeruginosa MTCC 7453, P.alcaligenes MTCC 493, P.cereus MTCC 7190, P.fluorescence MTCC 1749, P.putida MTCC 7525.) were obtained from the Microbial type culture collection, Chandigarh, India. The PHB producing capability of the organism was confirmed by Nile blue A and Sudan black staining method (Kitamara and Doi, Almost every product we buy most of the food we eat and many of the liquids we drink come encased in plastic packaging, which provides excellent protection for the product. It is cheap to manufacture and seems to last forever. Lasting forever, however, is proving to be a major environmental problem. Plastics are manufactured from numerous non-renewable resources like natural gas, coal, and oil. Plastics cannot be degraded (xenobiotic compounds) easily in the natural environment due to their long polymer molecules which are too large and too tightly bound.
The following are few items and the time required for their decomposition, Tin cans- 50 to 100 years., Aluminum Cans- 80 to 100 years., Glass Bottles- 1 million years., Newspapers- 25 to 50 years., Polystyrene- 1000’s of years., and Plastic bags- 400 years
A type of study on hard plastic is made with a molecule known as Bisphenol A, BPA. BPA like many other man made chemicals is now detectable in most people’s blood 1994).
Distruption of cells by chemical methods and PHB estimation (Slepecy and Law, 1961)
Nutrient broth was prepared in test tubes and inoculated with Pseudomonas cultures. The medium was incubated at room temperature for 24 -96 hours. PHB was estimated at every 24 hours interval. 5 ml of culture was taken and centrifuged at 10,000 rpm for 10 minutes at room temperature. Supernatant was discarded. The pellet was suspended in 2.5 ml of chloroform and it was incubated at 30 °C for 1 hour. The above contents were centrifuged at 1500 rpm for 10 minutes at room temperature. The upper hypochlorite phase, the middle chloroform containing undisturbed cells and the bottom chloroform phase with PHB was obtained. The upper and middle phases were separated from the chloroform phase using a micropipette. The contents were again centrifuged at 1500 rpm for 10 minutes at room temperature and the phase other than chloroform with PHB was removed carefully using micropipette. Concentrated sulphuric acid was added to the chloroform phase containing PHB, was then boiled at 100°C in a water bath for 10 minutes. The absorbance of the samples was read at 230 nm using UV Spectrophotometer.
a) By using Crotonic acid
Since PHB is converted to crotonic acid on heating with concentrated sulphuric acid, pure crotonic acid salt (Himedia, India Ltd) is used as a standard for estimation. Crotonic acid was taken in concentrations from 1-10 μg/ml and to this 5 ml of concentrated sulphuric acid was added. The tubes were heated at 100° C in a water bath for 10 minutes. The tubes were then cooled at 25 ° C and the absorbance was read at 230nm and a standard graph was plotted.
b) From Pseudomonas species
The above said standard procedure was been followed for the estimation of PHB from microbial source.In the place of crotonic acid microorganisms produced PHB will be replaced.
c)Effect of pH and temperature
24 hours old Pseudomonas species were inoculated into nutrient broth of different pH (5, 6,7and 8). The flasks were then incubated at 37° C for 48 hours and Nutrient broths were prepared and inoculated with 24 hours old Pseudomonas species. The flasks were incubated at different temperature (25, 30, 35 and 40) for 48 hours. The cells were harvested by centrifugation, disrupted by sodium hypochlorite and PHB accumulation was recorded.
PHB production and extraction in less expensive substrates
Food waste substrates such as (bagasse,wheatbran, whey),Industrial waste (Activated sludge) and Forestry residue (saw dust) were collected and were used for the PHB production.The PHB production by Pseudomonas species on different industrial waste were compared with PHB production in industrial waste incorporated with Nutrient broth. Pseudomonas species were inoculated into these different substrates and incubated for 48 hours at 37° C. After the incubation, the cells were harvested by centrifugation, disrupted using sodium hypochlorite and PHB was estimated.
Method of Extraction and analysis
Extraction of poly hydroxyl butyrate (PHB)
After 48 hours incubation at 37ºC, 10 ml of culture was taken and centrifuged at 8000 rpm for 15 min. The supernatant was discarded and the pellet was treated with 10 ml of sodium hypochlorite and the mixture was incubated at 30ºC for 2 hours. After incubation, the mixture was centrifuged at 5000 rpm for 15 min and then washed with distilled water, acetone, methanol respectively for washing and extraction. After washing, the pellet was dissolved in 5 ml of boiling chloroform and was evaporated the chloroform by pouring the solution on sterile glass tray and kept at 4º. After evaporation the powder was collected for further analysis.
Analysis of poly hydroxyl butyrate (PHB)
Pure PHB sodium salt (Sigma chemical Co. USA) was used as the standard to identify the PHB obtained from the Pseudomonas species.
Thin layer chromatography (TLC)
TLC plates were prepared by mixing 50 g of silica gel powder (chromatographic grade, Himedia India limitted) in 100 ml of distilled water .The gel was then cast into a thin layer over a glass plate .The PHB salt and PHB extracted from Pseudomonas species were individually dissolved in chloroform and were spotted on the plates .It was then developed by using a solvent system consisting of petroleum ether, acetone, and acetic acid in the ratio of 95:4:1. The plates were then kept in a saturated iodine chamber to render the lipid spots visible.
Isolation and identification of the fungal PHB –polymer degrader
Soil samples were collected from the garden. The fungi were isolated on Rose Bengal Agar. Samples were suspended by vortexing in sterile distilled water and allowed to stand for several minutes. The supernatants were then serial diluted, 0.1 ml from each dilution was plated onto the Rose Bengal Agar plates and incubated at room temperature for 2-7 days. The isolated fungi were maintained on Rose Bengal Agar slants.
Screening for PHB and PHB degradation
The polymer degrading ability of the isolated fungi was determined by different techniques. For the fungal isolates the basal medium was used which was supplemented with 0.01% peptone and 0.01% yeast extract, containing about 0.02% (w/v) of dry polymer (either PHB or PHB-co-HV) as a sole carbon source and solidified with 1% agar at pH 6.0.(Lee et al., 2005). The plates were incubated at room temperature for 1-3 weeks.
Bio control mechanisms of PHB
Isolation of vibrio species from Chicken sample
Spoiled chicken of 25 gms was weighed and taken .It was suspended in 250 ml of peptone water which gave a dilution of 10ֿ1 ,from which 1 ml was serially diluted upto 10ֿ6 .Spread plate technique was performed using TCBS medium . The plates were then incubated at 37° C for 24 hours.
PHB is used as biocontrol agent against Vibrio spp.(Vibrio infected aquaculture organisms)
Three marine fishes True pericula downfish (Amphiprion percula)were taken and 1000mg of PHB was added with 1000ml of sea water .After 24 hrs the fishes were challenged with Vibrio. Three fresh water fishes Lemon cichlid (Neolamprologus leleupi) were taken and 1000mg of PHB was added with 1000ml of fresh water. After 24 hrs the fishes were challenged with Vibrio.
Results
Identification of PHB producing Pseudomonas species by phenotypic methods
By culture test
Growth on Nutrient agar with 1 % Nile Blue A . (Ostle and Holt, 1982)
Pseudomonas species formed grey colored colonies on nutrient agar with 1% Nile Blue A indicated that they accumulate Polyhydroxybutrate .In differential staining revealed the presence of black sudanophilic granules within the pink cytoplasm when observed by using bright field microscope under oil-immersion
Estimation of Polyhydroxybutrate
a)By using crotonic acid
When PHB boiled with concentrated sulphuric acid crotonic acid was formed . The amount of PHB accumulated was estimated in an indirect method (in the form of crotonic acid ) and the absorbance was read at 230 nm by UV spectrophotometer. The readings obtained were plotted in standard graph of crotonic acid and the concentration of PHB was determined.
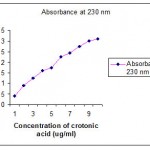 |
Figure 1
|
b)From Pseudomonas species
The intracellular storage granule was estimated after disruption of cells by using sodium hypochloride . During the reaction PHB was expressed in the form of crotonic acid and that was estimated . It was observed that PHB accumulation was on its peak at 48 hours and declined at 96 hours . This decline indicated the utilization of storage granule as carbon source . P.aeruginosa accumulated maximum amount (3.8μg /ml ) and P.alcaligens accumulated less amount (3.2μg / ml)
C)Effect of pH and temperature
The intracellular accumulation of PHB was maximum in P.aeruginosa (5.2μg/ml) and in P.alcaligens (4.9 μg /ml ) at pH 7 ,and maximum in P.aeruginosa (5.8μg/ml) and in P.alcaligens (4.7 μg /ml ) at 35° C , whereas the other Pseudomonas species accumulated less amount of PHB .
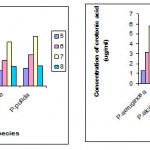 |
Figure 2
|
Estimation of PHB by Phenotypic studies by using less expensive substrates .
Food wastes
Baggase
Baggase incorporated with Nutrient broth the intracellular accumulation of PHB was maximum in P.aeruginosa (3.91μg/ml) and in P.alcaligens (3.6 μg /ml ) , whereas the intracellular accumulation of other Pseudomonas species was minimum .
In Baggase the intracellular accumulation of PHB was maximum in P.aeruginosa (6.1μg/ml) and in P.alcaligens (5.3 μg /ml ) , whereas the intracellular accumulation of other Pseudomonas species was minimum
Wheat bran
wheat bran incorporated with Nutrient broth the intracellular accumulation of PHB was maximum in P.aeruginosa (3.8μg/ml) and in P.alcaligens (3.1μg /ml ) , whereas the intracellular accumulation of other Pseudomonas species was minimum .
In wheat bran the intracellular accumulation of PHB was maximum in P.aeruginosa (6.7μg/ml) and in P.alcaligens (5.8μg /ml ) , whereas the intracellular accumulation of other Pseudomonas species was minimum .
Whey
Whey incorporated with Nutrient broth the intracellular accumulation of PHB was maximum in P.aeruginosa (3.609μg/ml) and in P.alcaligens (3.307μg /ml ) , whereas the intracellular accumulation of other Pseudomonas species was minimum .
In whey the intracellular accumulation of PHB was maximum in P.aeruginosa (6.95μg/ml) and in P.alcaligens (6.25μg /ml ) , whereas the intracellular accumulation of other Pseudomonas species was minimum .
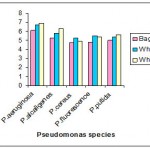 |
Figure 3
|
Industrial waste
Activated sludge
Activated sludge of 100 ppm incorporated with Nutrient broth the intracellular accumulation of PHB was maximum in P.aeruginosa (3.0μg/ml) and in P.alcaligens (2.8 μg /ml ) and in 200 ppm the intracellular accumulation in P.aeruginosa (3.31μg/ml) and in P.alcaligens (3.11 μg /ml) was maximum , whereas the other Pseudomonas species accumulated less amount of PHB.
In activated sludge of 100 ppm the intracellular accumulation of PHB was maximum in P.aeruginosa (5.2μg/ml) and in P.alcaligens (5.0 μg /ml ) and in 200 ppm the intracellular accumulation in P.aeruginosa (5.8μg/ml) and in P.alcaligens (5.1 μg /ml ) was maximum , whereas the other Pseudomonas species accumulated less amount of PHB .
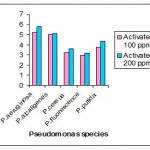 |
Figure 4
|
Forestry residue
Saw dust
Saw dust of 1 gm and 2 gms were incorporated with Nutrient broth the intracellular accumulation PHB was maximum in P.aeruginosa (3.452μg/ml and 4.871μg/ml) and in P.alcaligens (3.111 μg /ml and 4.253μg/ml) whereas the other Pseudomonas species accumulated less amount of PHB .
In saw dust of 1 gm and 2 gms the intracellular accumulation PHB was maximum in P.aeruginosa (4.92μg/ml and 5.502μg/ml) and in P.alcaligens (3.732 μg /ml and 4.603μg/ml) whereas the other Pseudomonas species accumulated less amount of PHB.
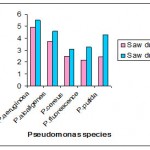 |
Figure 5
|
Extraction and analysis of accumulated PHB by Biochemical method
Thin layer chromatography (TLC)
Extracted PHB from Pseudomonas species and PHB sodium salt as standard were run on the column . The relative front(Rf) value of the standard and PHB isolated from 5 Pseudomonas species was calculated as below .
Rf value = Distance moved by the solute / Distance moved by the solvent .
Rf value of PHB standard = 13.2/17=0.776
Rf value of PHB of P.aeruginosa = 13.2/17=0.776
Rf value of PHB of P.alcaligenes = 13.2/17=0.776
Rf value of PHB of P.cereus =13.2/17=0.776
Rf value of PHB of P.fluorescens =13.2/17=0.776
Rf value of PHB of P.putida =13.2/17=0.776
The Rf value of the lipids aligned in the same position indicated that the extract contained PHB
Isolation and Screening of fungal isolates
Fungi (Aspergillus sp.) were isolated. In order to test the microbial PHB whether it can be degraded by another organism. The results shows that soil isolate Aspergillus sp have the high affinity towards PHB degradation within 3-6 days by observing the clear zone in the plate culture technique.
Bio control mechanisms of PHB
The isolated Vibrio spp. Made to infect the aquaculture fishes treated with PHB.The result shows that there is no mortality in PHB treatment, where as the fishes not treated with PHB shown mortality rate 25 % on third day after the infection and 45 % after fifth day of the infection. Gradually the mortality rate was increased after seventh day onwards.
Discussion
Plastics are recalcitrant or resistant , to microbial degradation because bacteria have not been exposed to them through the course of evolution . The high molecular weight of the compound provides durability and makes them resistant to biodegradation . The substitution of biodegradable plastics to non – biodegardable plastics is one of the major solutions to combat ecological problems presented by synthetic plastic. The first bacterial polymer discovered was polyhydroxyalkanoate in 1925 from B.megaterium by Lemoigne ,at Pasteur Institute . Majority of PHA is in the form pf PHB . High PHB production has been shown in a variety of different microorganisms including species of Pseudomonas , Bacillus, Azotobacter, Beijerinkia and Derxia.
Although there is considerable industries interest in PHA because of their potential as a biodegradable material their use is limited in many applications because they are more expensive than conventional plastics . Most of the carbon substrates available are pure alkanes , fatty acids and carbohydrates (Johanna et al .,2007). Among the 5 Pseudomonas species ,Pseudomonas aeruginosa and Pseudomonas alcaligenes accumulated maximum amount of PHB in nutrient broth at pH 7 (5.2μg/ml and 4.9μg/ml ) after 48 hours incubation at 35°C (5.8μg/ml and 4.7μg/ml).Dieter jendrossek et al .,(2007) carried out a similar study in Beijerinckia indica by using minimal medium and incubation temperature was 37°C for 72 hours .The growth parameters such as pH 7 and temperature 35°C were found to be optimum for the PHB accumulation in Pseudomonas aeruginosa and Pseudomonas alcaligenes .
Further studies included the formulation of a less expensive substrates for the accumulation of PHB . Food wastes like bagasse and wheat bran were used to study the PHB accumulation.Pseudomonas aeruginosa (6.1 μg /ml and 6.7μg/ml) and Pseudomonas alcaligenes (5.3 μg/ml and 5.8μg /ml)were accumulated maximum amount of PHB. He et al.,(1997) carried out a similar study in Pseudomonas coorrugata . Nutrient broth was supplemented with soya bean oil and 0.8 % of glucose and incubated at 28 °C for 52 hours and it produced 7.0 μg/ml of PHB.
Organic material has been hydrolysed into lower molecular weight compounds (Maede etal.,1994). Acid-hydrolysd saw dust mainly generates sugar monomers and small amount of nitrogen and phosphorous . The generation of these compounds enables the management of C:N ratio for improved bacterial biosynthesis of PHA (Tobella et al .,2007) . In saw dust of 1 gm and 2 gms ,Pseudomonas aeruginosa(4.92μg/ml and 5.502μg/ml) and Pseudomonas alcaligenes (3.73μg/ml and 4.603μg/ml ) were accumulated more amount of PHB.
Whey has a very strong polluting capacity, with a biological oxygen demand (BOD) of 40,000 to 45,000 mg/L (Hacking, 1988; Kemp and Quickenden, 1989). The production of microbial biopolymer(Schwartz and Bodie, 1985; Fu and Tseng, 1990; Flatt et al., 1992; Konicek et al., 1993). Azotobacter sp capable of accumulating PHAs (homo- and copolymers) in chemically defined media during growth without a nutrient limitation (Gonzalez- Lopez et al., 1997). In whey Pseudomonas aeruginosa (6.95 μg/ml) and Pseudomonas alcaligenes (6.28 μg/ml ) were accumulated more amount of PHB. In our present study concluded that the less expensive substrates produce more amount of PHB than the substrate incorporated with Nutrient broth.
The degradation study by a fungus indicated that they are biodegradable.Similar such studies were carried out in Rhizopus (S.V. Reddy M. Thirumala and S.K. Mahmood ).
Infections caused by luminescent vibrios can cause dramatic losses in aquaculture. These infections are often hard to treat with antibiotics because of the spread of resistant strains and therefore, alternative control strategies are urgently needed. So PHB can be used to protect the aquacultures against Vibrio infection.Inthis present investigation both marine and fresh water fishes and other aquaculture organisms were protected by using PHB.Similar such studies were carried out in aquaculture Artemia (larva of Nauplii) Defoirdt Tom, Dirk Halet , Patrick Sorgeloos, Peter Bossier and Willy Verstraete 1 Ghent.
Microbial source biopolymer not only replaces the synthetic polymer but it also have the high efficiency in disease controlling mechanism in marine and aquaculture organisms.Further work can be done in the area of specificity and immuno technological studies.
References
- Adams, A. 1991. Response of penaeid shrimp to exposure to Vibrio species. Fish Shellfish Immunol 1:59–70.
- Chen, G.Q., Konig, K.II. and Lafferty, R.M., 1991. Occurrence of PHA in the genus Bacillus. FEMS Microbiol. Letters, 84: 173-176.
- Doi, Y., 1990. Microbial polyesters. VCH Publishers, Inc, Yokohama, Japan, 33:62.
- Doi, Y., 1992. Microbial synthesis and properties of poly-hydroxyakanoates. A Publication of the materials research society, 17:39.
- Dieter jendrossek, K., 2007.PHB granules at the early stages of formation are localized close to the cytoplasmic membrane. Applied and Environmental Microbiology, 35:554– 576.
- He, N., Labuzck, S. and Radeeka, I., 1997. Isolation of PHA producing strains with industrial applications. J. Microbiol., 9(2): 95-102.
- Fiechler, A., 1990. Poly β hydroxyalkanoates as natural, Biocompatible and biodegradable polyesters. Springer – Verlag. New York, 77-93.
- Johanna,A.,Breso,M., and Silva,A., 2007. Biomacromolecule,5: 553.
- Kim, B.S., S.C. Lee, S.Y. Lee, H.N. Chang, Y.K. Chang and S.I.Woo, 1994a. Production of poly(3- hydroxybutyric acid) by fed-batch culture of Alcaligenes eutrophus with glucose concentration control.Biotechnol. Bioeng., 43: 892-898.
- Kim, B.S., S.C. Lee, S.Y. Lee, H.N. Chang, Y.K. Chang and S.I.Woo, 1994b. Production of poly(3- hydroxybutyric-co-3-hydroxyvaleric acid) by fed-batch culture of Alcaligenes eutrophus with substrate controlusing on-line glucose analyzer. Enzyme Micro.Technol., 16: 556-561.
- Lemoigne, M. C., 1926. Products of dehydration and of polymerization of hydroxybutyric acid, Bull Soc Chem. Biol., 8: 770-782.
- Maede,M.,Teresa,M. and Ricardo, G., 1994.Metobolic Engineering of poly-3 hydroxyalkanoates. Microbiology and Molecular biology. Revs., 21-53
- Ostle, A.., and Holt, J. G., 1982. Nile blue A fluorescent stain for poly-β-hydroxybutyrate. Appl. Environ. Microbiol., 2: 238-241.
- Slepecky, R. A. and Law, J. H., 1960. Synthesis and degradation of poly hydroxybutyric acid in connection with sporulation of B. megaterium. J. Bacteriol., 82: 25-32
- Slepecky R. A. and Law, J. H., 1961. Assay of PHB. J. Bacteriol., 82: 37-41.

This work is licensed under a Creative Commons Attribution 4.0 International License.





