Manuscript accepted on : December 31, 2009
Published online on: --
HPLC Method Development for Testosterone Cipionate in Bulk Drug and Oil-Based Injectables
Rakesh Kumar Gupta1, R.K. Roy1, Anurag1 and A.N. Jha2
1Dr.K.N.Modi Institute of Pharmaceutical Education and Research, Modi Nagar (India). 2Belco Pharma. , Bahadurgarh (India).
ABSTRACT: Isocratic liquid chromatographic method for the determination of testosterone cipionate (TC) in bulk drug and oil-based injectables have been developed and validated. Mobile phase methanol: water (90:10) was used for Testosterone Cipionate. For this method , a bonded-silica Luna 5μ C8 (2)100A (250 mm ª 4.6 mm, C 8, 5 micron.) (250C) column, a flow-rate of 1.2 ml min”1 and UV absorbance detection at 240 nm were used and separations up to base line were achieved. Prior to HPLC analysis, different dilution of bulk drug and sample preparation was required from oil-based injectables using the surfactant sodium dodecyl sulphate.
KEYWORDS:
HPLC; Validation; Testosterone cipionate; Oil-based injectables
Download this article as:| Copy the following to cite this article: Gupta R. K, Roy R. K, Anurag, Jha A. N, HPLC Method Development for Testosterone Cipionate in Bulk Drug and Oil-Based Injectables. Biosci Biotech Res Asia 2010;7(1) |
| Copy the following to cite this URL: Gupta R. K, Roy R. K, Anurag, Jha A. N, HPLC Method Development for Testosterone Cipionate in Bulk Drug and Oil-Based Injectables. Biosci Biotech Res Asia 2010;7(1). Available from: https://www.biotech-asia.org/?p=9798 |
Introduction
Testosterone is a steroid hormone from the androgen group. In mammals, testosterone is primarily secreted in the testes of males and the ovaries of females, although small amounts are also secreted by the adrenal glands. It is the principal male sex hormone and an anabolic steroid. In men, testosterone plays a key role in health and well-being as well as in osteoporosis. On average, an adult human male body produces about forty to sixty times more testosterone than an adult female body, but females are, from a behavioral perspective (rather than from an anatomical or biological perspective), more sensitive to the hormone1.Alkylation in the 17 position results in derivatives that are orally actives (e.g.methyltestosterone). Esterification in 17 position with organic acids results in derivatives, such as testosterone cypionate (TC), with improved oil solubility, employed for intramusculary administration in injectable forms. For this compound, the ratio of solubility between oil and water gives good correlated predictions of the ratios of solubility between blood and target organs2 . If consistent blood levels are desired then the shorter chain esters must be injected more frequently than longer ones. This leads to the greatest efficiency of the drug and the highest anabolic/androgenic ratio3.GC/MS was proposed for the determination of T esters in plasma and hair as a definitive proof of the administration of exogenous T 4-7. Analysis of T esters in chemicals, bulk materials and pharmaceutical preparations, has been investigated by gas chromatography / combustion /isotope ratio (GC/C/IRMS), based on the 13C/12C ratio8 . An alternative MS technique is HPLC/electrospray MS, which has been used for characterizing steroid esterified with long-chain fatty acids 9. A simple one-step extraction from oils or tablets was found to be an adequate sample preparation procedure for screening and quantitation of T esters by HPLC-UV 10. A general screening method by HPLC with UV–VIS particle beam mass spectrometry for the determination of anabolic steroids in oil-based injectables, water suspensions, dietary supplements, and herbal drugs marketed in the form of capsules or tablets has also been described 11. Also, the residues of AAS in misplaced injection sites and anabolic preparations are monitored by HPLC-UV-DAD 12.In addition, the analysis of testosterone esters in oil-based injectables has been described in the USP employing TLC and UV spectrometry for testosterone propionate and enanthate, and TLC and GC-FID for testosterone cipionate 13. Currently, it is possible to find a plethora of methods based on RP-HPLC for the determination of active ingredients in bulk drug and pharmaceuticals. Ghosh has described 1300 HPLC methods for hundreds of them. However, only a few of the proposed methods have been adequately validated 13-15.
Our aim is to develop and validate simple, rapid, sensitive, accurate, precise, reproducible and robust HPLC method for TC determination in bulk drug and oil-based injectables using a Luna 5µ C8 column and UV absorbance at 240 nm, have been determined. This method can be considered as an alternative to those reported by the most important pharmacopoeias for the quantitation of major component (TC) in bulk drug and oil-based injectables.
Experimental
Chemicals and reagents
TC (4-androsten-17 – (3-ciclopentyl-1-oxopropoxy)-3-one) working standard and sample injection supplied by Belco Pharma Ltd.(India) and Symbiotica Ltd(India). HPLC grade water and methanol (MeOH), sodium dodecyl sulphate, dichloromethane, acetonitrile were purchased from E. Merck India Ltd. Other chemicals were of analytical reagent grade.
Apparatus
The chromatography system consists of Sonicator- PCI MUMBAI (3.5 LITRES), Software Pump- LC SOLUTION, SHIMADZU LC-20 AT, UV-VIS Spectrophotometer- SHIMADZU-1601, HPLC SYSTEM LC 2010 AHT, Column- Luna 5 µ C8 (2) 100A.
Identification Test
Determination of λmax
λmax scanning in various solvent system namely dichloromethane, methanol, acetonitrile, water at various pH was done. The above solvent were also used in various ratio viz : dichloromethane : methanol (10 : 90, 20 : 80, 30 : 70, 40 : 60, 50 : 50, 60 : 40, 70 : 30, 80 : 20, 90 : 10 ) methanol : acetonitrile (10 : 90, 20 : 80, 30 : 70, 40 : 60, 50 : 50, 60 : 40, 70 : 30, 80 : 20, 90 : 10 ) methanol : water (60 : 40, 70 : 30, 80 : 20, 90 : 10 ) was employed to improve the sensitivity. In such combination, the composition selected was one in which the drug gave maximum absorbance. The final decision for using methanol: water (90: 10 v/v) as a solvent was on sensitivity, ease of preparation, suitability for drug contained estimation. The λmax was found to be 240nm.
Development and validation of HPLC method
Method development
Isocratic programming with methanol: water was taken into consideration. 90: 10 v/v of methanol: water gave appreciable separation. The chromatography conditions for the study includes chromatographic column, Luna (C8) with 5µm particle size. The mobile phase used methanol and water (v/v) and flow rate was 1.2ml/min. The column was maintained at ambient temperature and the eluant was monitored at a wavelength of 240 nm. The injection volume was 20µl.
Determination of λmax
A 100µg/ml of testosterone Cypionate drug solution prepared in methanol as solvent and scanned to determine the λmax methanol : water (90: 10) was selected based on sensitivity, ease of preparation and suitability of drug content estimation time and cost. The λmax found to be 240 nm. (Fig. 1)
Validation of developed HPLC method
Linearity
Linearity was established over of five different concentration of analyte. A stock solution of 500µg/ml was prepared in methanol and successively diluted to get sample 50-200 µg/ml of testosterone cypionate. Each concentration was injected in triplicate. The area counts against concentration were plotted and using linear regression; the slope, y-intercept and the co-efficient of co-relation within 95% confidence were determined.
Acceptance criteria
Co-relation co-efficient (R2) ≥ 0.999
Accuracy as recovery
Accuracy of an analytical method is the closeness of the test results obtained by that to the true values. This was determined in terms of percent recovery by applying the analytical procedure to the standard samples over a range of 80%, 100%, and 120% of the test concentration. In simple terms, the analytical procedure was applied to calculate the drug content in bulk drug to which known quantity of standard was added.
Preparation of stock solution
25 mg of finely powdered marketed raw material of testosterone cypionate was taken and diluted with methanol in 50ml volumetric flask, made up the volume and sonicated. The resulting concentration of test sample 500µg/ml of testosterone cypionate was used in the further study. Test concentration of working standard/sample solution is 100µg/ml.
Preparation of sample solution
Preparation of 80% sample solution
Taken 4ml of bulk drug stock solution in 25 ml volumetric flask and diluted with methanol, made up the volume. This solution was 80% sample solution. This solution was prepared, diluted and injected in triplicate.
Preparation of 100% sample solution
Taken 2ml of bulk drug stock solution in 10 ml volumetric flask and diluted with methanol, made up the volume. This solution was 100% sample solution. This solution was prepared, diluted and injected in triplicate.
Preparation of 120% sample solution
Taken 6ml of bulk drug stock solution in 25 ml volumetric flask and diluted with methanol, made up the volume. This solution was 120% sample solution. This solution was prepared, diluted and injected in triplicate.
Preparation of standard solution
25 mg of finely powdered reference standard of testosterone cypionate was taken and diluted with methanol in 50ml volumetric flask, made up the volume and sonicated. The resulting concentration of reference standard 500µg/ml of testosterone cypionate was used in the further study. Test concentration of reference standard is 100µg/ml.
Recovery percentage is calculated as
Acceptance criteria
Range 97.0% to 103.0%
Intra day precisions
Repeatability was performed on the same day by making repeat injection of samples ranging from 80µg/ml to 120 µg/ml for testosterone Cypionate. Stock solution of 500 µg/ml of testosterone cypionate was prepared and diluted to get concentration of 80µg/ml to 120 µg/ml for testosterone cypionate. Each dilution was injected in triplicate. The peak area for all the three injections was reported and the mean and RSD were calculated.
Acceptance criteria
%RSD≤1.5%
Range
The range was established from the linearity, accuracy and precision studies.
Specificity
Selectivity is the extent to which the analyte may be determined without interference from other components in a mixture, where impurities cannot be obtained as known standard, stress testing may be applied.
Robustness
Robustness is the reproducibility of the test results with respect to deliberate variations in the method parameters. A working solution of 100µg/ml for testosterone cypionate was taken and the following method parameters were changed independently of each other.
Mobile phase composition (±2%)
Flow rate (±0.2 ml/min)
pH
Injections were repeated thrice with change in the value of each parameter.
Acceptance criteria
%RSD≤2.0%
Result and discussion
HPLC Methods
Method development
To develop a precise, accurate, robust method for the determination of testosterone cypionate, different mobile phases and stationary phases were employed and the proposed chromatographic condition was found appropriate for the simultaneous determination.
Chromatographic condition
Chromatographic column used was a Luna (C8) with 5µm particle size. The mobile phase used methanol and water (v/v) and flow rate was 1.2ml/min. The column was maintained at ambient temperature and the eluant was monitored at a wavelength of 240 nm. The injection volume was 20µl.
System suitability
The chromatographic was performed on C8 column [Luna, C8 (2)100A], and 5µm particle size).The chromatographic parameters evolved are number of theoretical plates, asymmetry of the peak and tailing factor.
System suitability result are presented as
Testosterone cypionate
Theoretical Plates 12930.6
Asymmetry Peak 1.141
Tailing Factor 1.40
It was concluded that the developed method is the optimum according to the studies parameters. The value of number of theoretical plates was higher then the accepted value of 2000.The tailing factor another parameter of ICH guidelines as a factor to be controlled was within the limit.
Stability of solution
Both reference and the bulk drug and formulation sample solutions were stable for 48 hours. The assay % was under the limit.
Validation study
Linearity
It was conducted that proposed method was linear in the concentration rage from 50 to 200µg/ml for testosterone cypionate. The coefficient of correlation was found to be 0.9999 for testosterone cypionate (Table 1 and 2) and fig. 2
Table 1: Linearity table of Testosterone cypionate (bulk).
| Concentration (µg/ml) | Mean
Response (Area) |
Statistical analysis | |
| Testosterone Cypionate | Testosterone cypionate (Mean Area) | Testosterone cypionate | |
| 50 | 2015402 | Slope= 43665 | |
| 50 | |||
| 50 | |||
| 100 | 4055613 | Intercept (b)=204211 | |
| 100 | |||
| 100 | |||
| 150 | 6449646 | Correlation coefficient =0.9999 | |
| 150 | |||
| 150 | |||
| 200 | 8494838 | ||
| 200 | |||
| 200 |
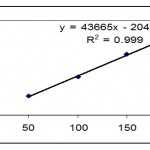 |
Figure 1
|
Table 2: Precision Readings: Intraday precision: this was done on the same day, % R.S.D was calculated.
| Conc of testosterone cypionate(µg/ml) | Testosterone cypionate
AUC |
Statistical analysis |
| Testosterone cypionate | ||
| 80
80 80 |
3239409
3247518 3243396 |
Mean = 3243441
% R.S.D= 0.12 |
| 100
100 100 |
4053561
4062401 4066879 |
Mean = 4060947
% R.S.D= 0.17 |
| 120
120 120 |
4853536
4858529 4863533 |
Mean = 4858532
% R.S.D = 0.14 |
Precision
In the study of intra assay precision and inter assay precision which have conducted on three different concentration levels ,three time same day and three time different day , showed a mean RSD of 0.14% & 0.16% for intra day precision and for inter day precision of testosterone cypionate . It shows that the proposed method is well précised (Table. 4-a, b).
Specificity
specificity test was done subjecting sample solution of testosterone cypionate to various stress condition like
0.1N HCI
0.1N NaOH
5% H2O2 solution.
The study was carried out to check the selectivity of the method. When treated the samples with 0.1N HCL, 0.1N NaoH and 5%H2O2 solution, no degraded peaks were obtained. After 24 hrs. RTs testosterone cypionate not affected by stress testing.
So it could be concluded that the method was selective.
Robustness
This was done by small deliberate changes in the chromatographic conditions. The factors selected were pH, flow rate and % methanol in the mobile phase. The mean of retention time and S.D were calculated and were found to be 7.4±0.062 of testosterone cypionate. The low level of S.D values indicates that the selected factors remained unaffected by variations of these parameters. (Table. 5)
Table 3: Robustness study.
| S.N. | Chromatographic conditions | Level | Testosterone cypionate
retention time |
| A | Flow rate(ml/min) | ||
| 1.0 | -2 | 7.512 | |
| 1.2 | 0 | 7.442 | |
| 1.4 | +2 | 7.392 | |
| Mean±S.D | 7.5±0.065 | ||
| B | % of methanol | ||
| 88 | -2 | 7.521 | |
| 90 | 0 | 7.443 | |
| 92 | +2 | 7.389 | |
| Mean±S.D | 7.5±0.067 |
Limit of Detection (LOD)
The detection limit was calculated by the following formula
LOD = 3.3σ/S
Where σ = the standard deviation of the response
S = the slope of the calibration curve
The LOD was calculated as 5 µg/ml of testosterone cypionate.
Limit of Quantification (LOQ)
The quantification limit was calculated by the following formula
LOD = 10σ/S
Where σ = the standard deviation of the response
S = the slope of the calibration curve
The LOQ was calculated as 15 µg/ml of testosterone cypionate.
Table 4: Assay of pharmaceutical formulation of testosterone cypionate injection (cypiobolic injection) Batch no. MR001c1 by developewd method
| S. NO. | Conc.(µg/ml) | % Assay | Mean % assay |
| 1 | 100 | 98.25 | |
| 2 | 100 | 100.01 | 98.5 |
| 3 | 100 | 98.70 |
Limit of detection & Limit of quatitation
Limit of detection & Limit of quatitation was calculated by the method which was based on standard deviation (SD) of the response and the slope (S) of calibration curve at level approximating the LOD and LOQ, LOD = 3.3 S.D/S and LOQ = 10 S.D/S of testosterone cypionate
Assay determination of testosterone cypionate from injection dosage form
The developed method was used in assay of commercially available injections containing 20 mg/ml of testosterone cypionate. Prior to HPLC analysis, sample preparation was required from oil-based injectables using the surfactant sodium dodecyl sulphate in methanol: water (90:10). The solution was sonicated for 30 min. Double filter it. .Placebo samples were prepared by mixing and homogenizingthe excipients of injectables, and processed in a similarway to the pharmaceuticals.
The assay value obtained was 99.25 and 100.01that was found within limit (97 to 103%).
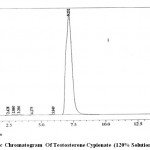 |
Figure 2 : Hplc Chromatogram Of Testosterone Cypionate (120% Solution)
|
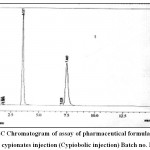 |
Figure 5: HPLC Chromatogram of assay of pharmaceutical formulation of testosterone cypionates injection (Cypiobolic injection) Batch no. MR1C1.
|
Conclusions
The proposed RP-HPLC method for the quantification of testosterone cypionate is simple, reliable and selective providing satisfactory accuracy and precision with lower limits of detection and quantification. Moreover the shorter duration of analysis for testosterone cypionate make this developed method suitable for routine quantitative analysis in bulk drug and pharmaceutical dosage forms. The recoveries achieved are good by applying this method.
References
- L.A. Kaplan, A.J. Pesce (Eds.), Clinical Chemistry, C.V. Mosby, St.Louis, MO, 1989.
- K.C. James, P.J. Nicholls, M. Roberts, J. Pharm. Pharmaceut. 21 (1969) 24–27.
- www.mesomorphosis.com/articles/pharmacology/anabolic-steroidesters.htm.
- J. Segura, S. Pichini, S.H. Peng, X. De la Torre, Forensic Sci. Int.107 (2000) 347–359.
- P.K. Mueller, J. Grosse, R. Lang, D. Thieme, J. Chromatogr. B 674(1995) 1–11.
- X. de la Torre, J. Segura, A. Pollettini, M. Montagna, J. Mass Spectrosc.30 (1995) 1393–1404.
- Y. Gaillard, F. Vayssette, A. Rolland, G. Pepin, J. Chromatogr. B735 (1999) 189–205.
- C.H.L. Shackleton, H. Chuang, J. Kim, X. de la Torre, J. Segura,Steroid 62 (1997) 523–529.
- M.J. Walters, R.J. Ayers, D.J. Brown, J. AOAC 73 (1990) 904–926.
- M.Z. Mesmer, R.D. Satzger, J. Chromatogr. Sci. 35 (1997) 38–42.
- A.Daxenberger, I.G. Lange, K. Meyer, H.H.D. Meyer, J. AOAC 83(2000) 809–819.
- The United States Pharmacopoeia, 28th ed., The United States Pharmacopoeial Convection, Rockville, MD, 2005.
- 762 R. Gonzalo-Lumbreras et al. / Journal of Pharmaceutical and Biomedical Analysis 38 (2005) 757–762
- M.K. Ghosh, HPLC Methods for Drug Analysis, Springer-Verlag,Berlin, 1992.
- J.M. Green, Anal. Chem. 68 (1996) 305A–309A.International Conference on Harmonization (ICH), Draft Guideline on Validation of Analytical Procedures, Federal Register 60 (1995)11260–11287.

This work is licensed under a Creative Commons Attribution 4.0 International License.






