Manuscript accepted on : January 15, 2010
Published online on: --
Heat Transfer Strategies in Itaconic Acid Production by Using Aspergillus Terreus MTCC 479
V. Meena
Department of Chemical Engineering, A.U. College of Engineering, Andhra University, Visakhapatnam India.
Corresponding Author E-mail: meena_sekhar09@yahoo.co.in
ABSTRACT: Itaconic acid was best produced by fungal species than bacterial species. The Aspergillus terreus were known to be the best species for itaconic acid production among the different fungal species studied. However, there was no comprehensive study on using latest technologies for increasing the productivity at industrial level and it was not properly established. For increasing the productivity, the influence of starting substrate concentration, the optimum timing of additional substrate and the possibility of semi-continuous fermentation were analyzed in a bench-top fermentation. In the present study we can analyse the requirements as follows: The total heat requirement for 3lt bench top fermentor - 103.16 kcal/ hr. Design of sparger – 10holes with 3mm diameter having 5mm pitch and Diameter of the shaft required – 13mm for the maximum yield of itaconic acid of 27 g/lt under optimized conditions by A.terreus.
KEYWORDS: Heat requirement; Itaconic acid; Shaft; Sparger.
Download this article as:| Copy the following to cite this article: Meena V. Heat Transfer Strategies in Itaconic Acid Production by Using Aspergillus Terreus MTCC 479. Biosci Biotech Res Asia 2010;7(1) |
| Copy the following to cite this URL: Meena V. Heat Transfer Strategies in Itaconic Acid Production by Using Aspergillus Terreus MTCC 479. Biosci Biotech Res Asia 2010;7(1). Available from: https://www.biotech-asia.org/?p=9499 |
Introduction
Organic acids with wide applications in various fields are made from living cells commercially. Organic acids like citric acid, gluconic acid, itaconic acid and lactic acids are manufactured by means of such large-scale bioprocesses. Among them, the itaconic acid (Methelene Butanedioic acid common synonyms: Methylene succinic acid, 3-carboxy-3-butanoic acid, propylenedicarboxylic acid) is the most promising one.
Itaconic acid is a colorless crystalline carboxylic acid obtained by the fermentation of carbohydrates. Itaconic acid was discovered by Baup (1837) as a thermal decomposition product of citric acid. The biosynthesis by fungi from carbohydrates was first reported by Kinoshita (1932), who isolated itaconic acid from the growth medium of an osmophilic fungus, Aspergillus itaconicus. Later, other fungal strains, mainly of the species Aspergillus terreus, were found to be more suitable. At the Northern Regional Research Laboratory (NRRL) of the U.S. Department of Agriculture in Peoria, Illinois, a screening programme of more than 300 strains identified the most published strain, A. terreus NRRL 1960 (Lockwood and Reeves, 1945). Attempts were also made to develop a biotechnical process for itaconic acid production (Nelson et al., 1952; Pfeifer et al., 1952). Later, optimized industrial processes were established providing the limited market with itaconic acid. The prominent developments in itaconic acid production (batch fermentation, free suspended biomass) took place before 1966 (Petuchow et al., 1980). Over the next 15 years, the interest in itaconic acid production declined, as indicated by the few publications during this time. Since the early 1980s, there has been increasing concern regarding sustainability, environmental conservation, renewable resources and rising energy costs.
Heat transfer design of fermenter :-
Heat input
i > By air
volume of air at NTP = 0.002 x 60
= 0.12 m3 /hr
![]()
Air inlet temp = 70oC
Air outled temp = 25oC
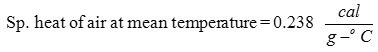
Heat lost by air = in Cp ∇ T
=0.155 x 0.238 x 2.203 x 45
=3.665 lb-cal / hr
![]()
= 3.5 x 0.002
=0.007 kw
iii> Heat input due to agitation = 7.5% (power input by impeller)
= 0.075 x 0.1864
= 0.01398 kw
Total = (i) + (ii) + (iii)
![]()
= 43.457 lb-cal/hr
Heat output
By evaporation of water
Po = Vap pressure of water at 25oC = 24 mm Hg
P = Air pressure = 1010 mm Hg
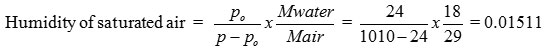
Quantity of dry air = 0.155 Kg/hr
∴Quantity of water evaporated = 0.155 x 0.015 11
=2.342 x 10-3 Kg/hr
= 5.159 x 10-3 lb/hr
Latent heat of water at 25oC = 583 Cal
∴Quantity of water = 5.159 x 10-3 x 583
= 3.0077 lb cal / hr
= 1.365 Kcal/hr
Heat removed by cooling water = 43.452 – 3.0077
= 40.4443 lb-cal/hr
Cooling water temperature = 18oC
If cooling water exit temperature = 22oC
![]()
Sp Gravity of water at 20oC = 0.997
![]()
If water velocity = 3ft/sec
Area of crops section required for flow
![]()
= 0.002166 in2
= 2.166 x 10-3 in2
![]()
![]()
= 56.8 x 103 in2
![]()
Heat transfer coeffect
O. d = 0.405”
I . d = 0.269”
Out side surface = π do
= π (0.405)
= 1.272 in
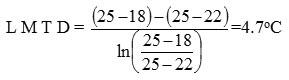
Calculation of coil inside film coeffct
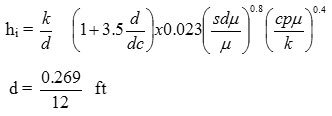
dc = dia of coil = 75 mm = 2.95”
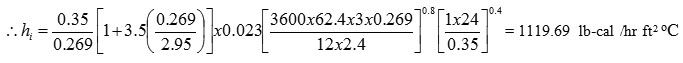
Calculation of coil outside film coeffct
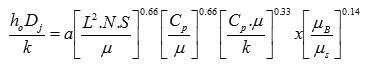
Dj = Vessel diameter
![]()
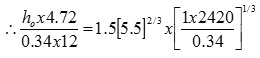
ho = 77.73 lb cal/hr. ft2 – oC
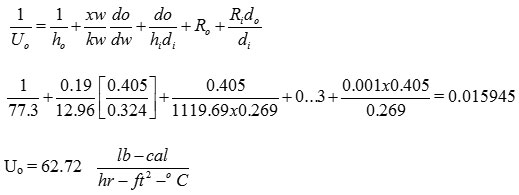
This value lies between 50 – 150
So, this pipe serve the purpose
Q = U A Δ T
40.449 = 62.72 A (4.7)
A = 0.1372 ft2
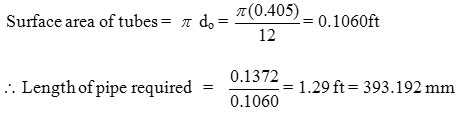
Length of pipe required =
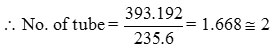
∴Two sets of coils with 2 turns each is used coil diameter = 75 mm, pitch = 5mm
Chemical Engineering design of fermenter
Pressure drop across coils
u = 3 ft /sec
= 3 x 3600 ft/hr
d= 0.269 dc = 75 mm =2.95”
NRe =
= 6242.57

= 9294.107
NRe crit > NRe
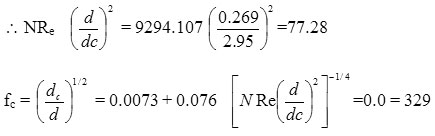
=0.0 = 329
fc = 0.00993
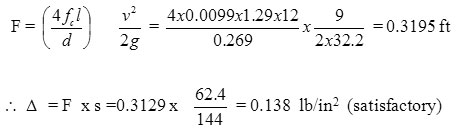
Heat balance over fermenter daten temp (0oC)
I› Heat input
Air
In = 0.155 kg/hr
T1 = 0oC
T2 = 70oC
Cp = 0.238 cal/g –oC
∴Qair = in cp T
= 0.155 x 0.238 x 70
= 2.5823 Kcal/hr
2> Fermentation & Agitation = 0.007 + 0.01398
= 0.02098 kw
= 0.02098 K J/sec
![]()
Cooling water
In = 4.589 kg/hr
Cp = cal/g – oC
T2 = 18oC
T1 = 0oC
∴Q = in CP T
= 4.589 x 1 x 18
= 82.602 Kcal/hr
Heat Output
1) Air
in = 0.155 Kg/hr
Cp = 0.238 cal/g-oC
T2 = 25oC
T1 = 0oC
∴Qair = in CP T
= 0.155 x 0.238 (25-0)
= 0.92225 Kcal/hr
Evaporation = 1.365 Kcal/hr
Cooling water
in = 4.589 kg/hr
T2 = 22oC
∴Q = 4.589 x 1 x 22
= 100.958 Kcal/hr
Table 1: Heat balance
| I/P Heat (Kcal/hr) | O/P Heat (Kcal/hr) | ||
| 1. Air | 2.5823 | Air | 0.922 |
| 2. Fermentation +Agitation | 17.98 | Evaporation | 1.365 |
| 3. Cooling water | 82.602 | Cooling water | 10.96 |
Total = 103. 1643 Total = 103.25
∴This completes the heat transfer design of fermenter
Design of shaft
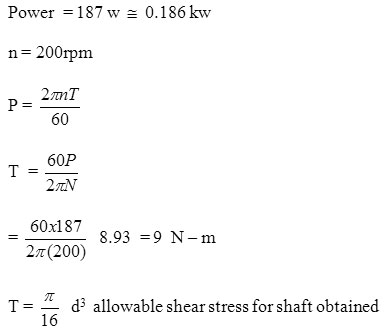
= 70 MPa
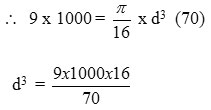
d = 12.7 mm
![]()
Mechanical design
Design of sparger
Height of liquid = 216 mm = 0.7 ft
![]()
Total pressure at the bottom of vessel = 5+0.32
= 5.32 lb/in2
Air pressure P1 = 14.7 x 2 = 29.4 lb/in2 (Abs)
Pressure at bottom P2 = 5.32 + 14.7 = 20.02 lb/in2 (Abs)
The velocity of flow
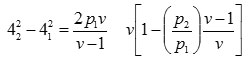
v = 1.4
u1 = 0
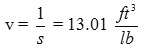
s = 1.29 Kg/m3 = 0.076 lb/ft3
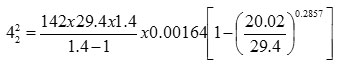
42 = 5.053 ft/sec
Area required for this flow
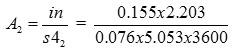
=0.246 x 10-3 ft2
=0.0228 x 10-3 m2
= 22.8 mm2
considering losses
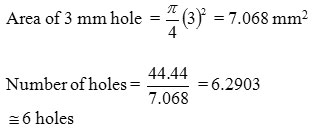
10 holes with 3 mm dia having 5 mm pitch
Welding joints
Shaft diameter = 13 mm
Number of bolts = 4.Cover plate thickness = 4 mm
Results and Discussion
The total heat requirement for 3lt bench top fermentor – 103.16 kcal/ hr. Design of sparger – 10holes with 3mm diameter having 5mm pitch. Diameter of the shaft required – 13mm
Production of Itaconic acid in Batch Fermentation (bench top fermentor):
An investigation has been done to study the maximum production of itaconic acid using different configuration reactors with a configuration volume of 100 ml and 2 batch reactors. Aspergillus terreus species were found to give a maximum yield of itaconic acid in 100 ml batch reactor than 2 reactor indicating that the 100 ml reactor was most suited for the production of itaconic acid. A. terreus has produced maximum itaconic acid of 27 g/lt (Figure) under optimized conditions (Table). The present observed results were found to be in consonance with the reports of Prucssc et al., (1998).
Table 2: Itaconic acid production using 100ml & 2 Lt batch reactor by A. terreus.
| S.NO. | Time
(hrs) |
Itaconic acid Concentration (g/lt) | |
| 100ml | 2 Lt | ||
| 1 | 0 | 0.00 | 0.00 |
| 2 | 24 | 1.04 | 1.03 |
| 3 | 48 | 5.13 | 4.93 |
| 4 | 72 | 8.97 | 8.05 |
| 5 | 96 | 13.14 | 13.09 |
| 6 | 120 | 27.00 | 26.40 |
Table 3: Anova: Two-Factor With out Replication
| Summary | Count | Sum | Average | Variance |
| Row 1 | 2 | 0 | 0 | 0 |
| Row 2 | 2 | 2.07 | 1.035 | 5E-05 |
| Row 3 | 2 | 10.06 | 5.03 | 0.02 |
| Row 4 | 2 | 17.02 | 8.51 | 0.4232 |
| Row 5 | 2 | 26.23 | 13.115 | 0.00125 |
| Row 6 | 2 | 53.4 | 26.7 | 0.18 |
| Column 1 | 6 | 55.28 | 9.213333333 | 100.0411867 |
| Column 2 | 6 | 53.5 | 8.916666667 | 96.28694667 |
Table 4:
| Source ofVariation | SS | df | MS | F | P-value | F crit |
| Rows | 981.2802 | 5 | 196.25604 | 2722.249 | 1.403E-08 | 5.05032 |
| Columns | 0.2640333 | 1 | 0.264033333 | 3.662382 | 0.1138396 | 6.60789 |
| Error | 0.3604667 | 5 | 0.072093333 | |||
| Total | 981.9047 | 11 |
Anova
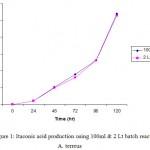 |
Figure 1: Itaconic acid production using 100ml & 2 Lt batch reactor by terreus.
|
Summary and Conclusion
Recent advancements in the field of industrial biotechnology have led to the development of successful organic acids, which is being used in the production of biodegradable plastics. Itaconic acid is made use as a co-monomer at a level of 1–5% for certain polymer products. It is also important as a constituent for the fabrication of synthetic fibers, coatings, adhesives, thickeners, and binders.
So the power requirement for the fermentor to produce maximum itaconic acid production carried in 3lt benchtop fermentor. A. terreus has produced maximum itaconic acid of 27 g/lt under optimized conditions.
References
- Bailey J and Ollis D. F. (1986) Biochemical Engineering Fundamentals ii Ed. Mc Graw-Hill New York.
- Bajaj P, Agarwal AK, Dhand A and Kasturia N. In: (2000) Flame retardation of Acrylic fibers. Polymer Reviews. 40: 309-337.
- Batti M, and Schweiger LB. (1963) US-Patent 3078 217 to Miles Laboratories: Process for the production of itaconic acid.
- Bentley R and Thiessen, C.P. (1957 b) Biosynthesis of itaconic acid in Aspergillus terreus. II. Early stages in glucose dissimilation and the role of citrate Bio. Chem 226:689-701.
- Berge’s Manual of Systematic Bacteriology, Springer US (2007).
- Butterworth (1973) cited in Willke Th and Vorlop KD. (2001) Biotechnological
- production of itaconic acid. Applied Microbiology and Biotechnology. 56: 289-295.
- Cargil (2000) Speeches http://www.cargill.com/today/speeches/00_6_5 micek.htm
- Cros P. and Schneider D. (1993) US-Patent 5 231 016 (to Rhone Poulene): Microbiological production of itaconic acid.
- Horitsu II, Xiao R, Nakamura Y and Kuwai K. (1983) Some factors affecting production of itaconic acid by Aspergillus terreus immobilized in polyacrylamide gels. Eur. J. Appl. Microbiol. Biotechnol. 18:358-360.
- Kazutoyo, Shibaba S, Jia SR, Park Y and Okabe M. (1997) Efficient itaconic acid production from raw corn starch. J. Ferm. Bioeng. 84: 375-377.
- Prucssc U, Fox B, Kirchhoff M, Bruske F, Breford J and Vorlop KD. (1998) The jet-cutting method as a new immobilization technique for spherical heat production. Biotechnol. Tech. 12: 105-108.

This work is licensed under a Creative Commons Attribution 4.0 International License.





