Manuscript accepted on : April 16, 2010
Published online on: 28-06-2010
S. R. Kazemi Nezhad*, M. Asoodeh Sarshoori1 and S. Daneshmand
Department of Genetics, Faculty of Science, Shahid Chamran University of Ahvaz, 65355-141 Iran.
Corresponding Author E-mail:kazemi_reza@scu.ac.ir
ABSTRACT: Glucose-6-phosphate dehydrogenase (G6PD) deficiency is one of the most frequent genetic enzymatic disorder in humans. G6PD is inherited as an X-linked recessive gene and encodes a vital housekeeping enzyme. The predominant variant of G6PD is named Mediterranean, often associated with Favism. Most G6PD-Mediterranean subjects also have a silent transition (C1311T). In the present study 1065 simple randomly selected blood samples of male donors from Ahvaz Blood Bank, using fluorescent spot test to determine G6PD deficiency in Khuzestan province. G6PD deficiency was found in 126 samples, 70 out of 126 samples were G6PD Mediterranean and the rest of them were shown other variants of G6PD deficiency including Chatham (19 samples), Cosenza (5 samples) and undefined G6PD gene mutation (32 samples). The association between C1311T silent polymorphism in all the G6PD deficiency samples and 70 male controls in Khuzestan province in Iran were studied. Screening was carried out by PCR-RFLP method. Prevalence of C1311T polymorphism was 92.8% among 70 Mediterranean samples and 15.7% among control group respectively. No patient was found for C1311T polymorphism in non-Mediterranean G6PD deficiency group. Statistical analysis showed a strong association between C1311T polymorphism and G6PD-Mediterranean (P<0.001). However, association between C1311T polymorphism and non-Mediterranean G6PD deficiency did not confirm.
KEYWORDS: G6PD-Mediterranean deficiency; C1311T polymorphism; Khuzestan Province; Iran
Download this article as:| Copy the following to cite this article: Nezhad S.R.K ,Sarshoori M.A, Daneshmand S. Association study between C1311T silent polymorphism and Mediterranean glucose-6-phosphate dehydrogenase in deficient Males in Khuzestan Province, Southwest Iran.Biosci Biotechnol Res Asia 2010;7(1) |
| Copy the following to cite this URL: Nezhad S.R.K ,Sarshoori M.A, Daneshmand S. Association study between C1311T silent polymorphism and Mediterranean glucose-6-phosphate dehydrogenase in deficient Males in Khuzestan Province, Southwest Iran.Biosci Biotechnol Res Asia 2010;7(1). Available from: https://www.biotech-asia.org/?p=8771 |
Introduction
Glucose-6-phosphate dehydrogenase deficiency (G6PDD) is one of the most common inherited disorder of red blood cell in human affecting more than 400 million people worldwide. G6PDD is caused by defects in g6pd gene and results in a number of different hemolytic anemias due to exposing to some oxidative agents.1 G6PD enzyme catalyses the first step of the pentose phosphate pathway and provides cells with required NADPH for biosynthesis and protecting them against oxidative stress, therefore is vital for cell survival.2 Since G6PD is the only source of NADPH generating enzyme in erythrocytes and the important function of this enzyme is detoxification of oxidative agents, erythrocytes are much more sensitive to lack or deficiency of this enzyme rather than other tissues.3
G6PD is known as one of the most polymorphic enzymes in human with respect to its biochemical and genetic character.1 Approximately all g6pd mutations occur in coding region and mainly result in single amino acid substitutions.4 No large deletion or frameshift mutation have been reported in this gene until now. This confirms that a total lack of G6PD is incompatible with life.
The G6PD gene located at chromosome Xq28, consists of 13 exons, and encodes a housekeeping enzyme expressing in all body tissues.4 Since G6PDD is an X-linked recessive disorder therefore it is more frequent in males than females. On the other hand distribution of G6PDD is highly correlated with the distribution of current or past malaria endemicity, because, G6PDD confers a reduced risk of infection by the plasmodium parasites.5,6
Some clinical manifestations of G6PD deficiency are chronic non-spherocytic hemolytic anemia, neonatal jaundice and acute hemolytic anemia related to infection, ingestion of Fava Bean (Favism) or some chemical agents or medicine.1 More than 380 different variants have been identified so far. 7,8A predominant variant of G6PD named Mediterranean is often associated with Favism. G6PD Mediterranean deficiency mutation causes C-T transition at position 563, converting Serine to Phenylalanine. The most G6PD-Mediterranean subjects also have a silent C>T transition (without amino acid replacement) at nucleotide position 1311 in exon11.9
Khuzestan Province (site of study) is located in the southwest of Iran. It borders Iraq in the west and Persian Gulf in the south. Most Iranian Arabs live in Khuzestan regions.
Materials and Methods
In this study 1065 simple randomly selected blood samples of male donors from blood bank center of Ahvaz were gathered. All samples were collected with written informed consent. Peripheral blood samples were collected in 0.5 M EDTA solution (Sigma) and kept at -70ºC.
G6PD deficiency diagnostic test for screening was done by fluorescent spot method (Sigma Diagnostics, Germany). Diagnosis of G6PD deficient samples using this test is based on observation of fluorescent light resulted by production of NADPH by G6PD enzyme, under UV light. This semi-quantitative assay is reliable and highly sensitive which classifies a sample simply as “normal” or “deficient”.10
Genomic DNA was extracted from white blood cells of the samples by standard method of DNA extraction kit procedure (High Pure) from Roche Molecular Biochemicals, Switzerland. The association between C1311T silent polymorphism in the G6PDD samples and 70 male controls in Khuzestan province in Iran were screened. DNAs of total samples were amplified for C1311T silent polymorphism using PCR. PCR reaction was performed using F-Med (5΄-TGT TCT TCA ACC CCG AGG AGT-3΄) and R-Med (5΄-AAG ACG TCC AGG ATG AGG TG A TC-3΄) primers to amplify the exon 11 and flanking regions, involving C1311T silent polymorphism. The PCR reaction was carried out for 35 cycles (each cycle consisted of 30 seconds with the following temperatures: denaturation: 94, annealing: 58 and extension: 72). Using 0.5 unit of Taq DNA polymerase (Fermentas GmbH, Germany) in a final volume of 25 μL. Then the PCR products were run on 1.5% agarose gel to verify the fidelity of PCR reaction. The C1311T silent polymorphism creates a new Bcl-1 enzyme (New England Biolabs. Ltd, Hitchin, UK) recognition site, so PCR products were digested with 0.7 unit of this restriction enzyme following PCR amplification for 16 hours at 55ºC. The products were run on 3% agarose gel to detect the mutation.
Results
G6PD deficient subjects were detected, using fluorescent spot test. G6PD-deficiency was found in 126 male samples which 70 samples were G6PD-Mediterranean and rest of them (56 samples) were other kind of G6PDD including Chatham, Cosenza and unknown G6PDD mutation.
Subsequently, 70 G6PD Mediterranean males, 70 controls and 56 other kind of G6PDD (Chatham, Cosenza and undefined G6PD deficiency mutation) were analyzed by PCR-RFLP method to characterize C1311T silent polymorphism.
A 203 bp fragment involving exon 10-11 was amplified from genomic DNA by PCR with F-Med and R-Med primers. Products were digested with Bcl-1 enzyme to detect C1311T silent polymorphism. The DNA with this mutation will be cleaved at the new recognition site, then a new fragment will be expected. After Bcl-1 digestion the normal samples with no C1311T silent polymorphism showed 203 bp fragments (59 samples) but normal samples with C1311T silent polymorphism showed 180 bp fragments (11 samples). Therefore 203 bp band for G6PD Mediterranean with no C1311T silent polymorphism (5 samples) and 180 bp for G6PD Mediterranean with C1311T silent polymorphism (65 samples, Table 1) subjects detected on agarose gel (Fig. 1). The 56 samples (other kind of G6PDD including Chatham, Cosenza and undefined mutation) which did not have C1311T polymorphism, showed 203 bp fragments. In this study we came out with the following results, illustrated in Table 2.
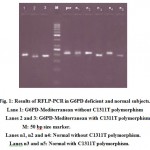 |
Figure 1: Results of RFLP-PCR in G6PD deficient and normal subjects.
|
Lane 1: G6PD-Mediterranean without C1311T polymorphism
Lanes 2 and 3: G6PD-Mediterranean with C1311T polymorphism.
M: 50 bp size marker.
Lanes n1, n2 and n4: Normal without C1311T polymorphism.
Lanes n3 and n5: Normal with C1311T polymorphism.
Table 1: Frequencies of polymorphism mutation nt 1311 in G6PD mutations
| Mutations | 1311C | 1311T | Total number |
| Mediterranean | 5 (7.2%) | 65 (92.8%) | 70 |
| Chatham | 19 (100%) | – | 19 |
| Cosenza | 5 (100%) | – | 5 |
| Another kind of G6PDD for mutation | 32 (100%) | – | 32 |
Table 2: Prevalence percent of C1311T polymorphism in G6PD-Meditrranean and normal subjects
| Total number | with C1311T polymorphism | without C1311T polymorphism | Subject |
| 70 | 65 (92.8%) | 5 | G6PD- Med |
| 70 | 11 (15.7%) | 59 | Control |
Table 3: Comparison prevalence percent between G6PD-Mediterranean and C1311T polymorphism in different area
| Area | Accompaniment percent |
| Mediterranean countries | 95.4 |
| Arabian countries
Iran |
95 |
| -Kermanshah | 90.3 |
| -Khuzestan | 92.8 |
Discussion
More than 130 different mutations have been described for the g6pd gene. The frequency of G6PD deficiency in the Middle East varies widely, ranging from 1% for Egyptian to 11.5% for some ethnical groups of Iran.11 According to the report of WHO the overall incidence of G6PD deficiency among the Iranian population was, 10%-14.9%.12
G6PD Mediterranean is the most prevalent G6PD deficient variant in many Middle East and Iran neighboring countries such as Turkey,13 Pakistan,14 India,15 Bahrain,16 Kuwait,17 Oman,18 Iraq,19 Saudi Arabia20 and the countries around the Mediterranean Sea.2 According to previous studies of g6pd gene in different provinces of Iran, G6PD Mediterranean has the most frequency among the other variants.21
The malaria parasite has been prevalent and endemic in some regions of Iran in the past or currently. On the other hand some g6pd variants such as G6PD Mediterranean decrease infection risk of malaria, thus this disorder has a high incidence rate in these regions.13
Results in the present study showed that the most G6PD Mediterranean subjects also have the silent C to T transition at the nucleotide position 1311 in exon 11 of g6pd gene.
Our finding showed 65 out of 70 individuals with Mediterranean-G6PD have C1311T mutation (Table 2), this finding indicates a strong association between C1311T polymorphism and G6PD Mediterranean deficiency in Khuzestan population (p<0.001).
However both G6PD Chatham and G6PD Cosenza were linked to the 1311C polymorphism. It is well established that there is a marked linkage disequilibrium between silent polymorphic sites with coding sequence polymorphisms.5 The Mediterranean mutation in Europe and Middle East is associated with a silent C>T transition at nucleotide position 1311. However, in the Western coast of south Italy and in India this mutation is associated with 1311C.22-24
The findings in this study indicate that G6PD Mediterranean mutation is of different origin in the Middle East, compared to other parts of Asia including India (Table 3).
The G6PD Mediterranean mutations with haplotype 1311C could be the result of either an interallelic crossover or population admixture. Also, the presence of strong association of the G6PD Mediterranean mutation and the presence of the polymorphism nucleotide 1311 C>T in the Khuzestan population demonstrate that the presence of this mutation may be the result of migrations that have taken place through the history of Iran.
Chatham and Cosenza chromosomes in the present study had 1311C haplotype. On the other hand in five Chatham chromosomes from Kuwait and one from Oman, 1311C haplotype was found. However in two Chatham chromosomes from Algeria and Oman there were the 1311T haplotype.25 In conclusion, finding the Chatham mutation on different chromosomal background could be the result of recombination or an independent origin of the mutations.
Acknowledgments
We would like to thank all patients who agreed to provide samples for this study. This research was supported by a grant from the Shahid Chamran University of Ahvaz, Khuzestan Province; grant no. 3627. The authors declare that they have no conflict of interests.
References
- Beutler E., G6PD Deficiency, Blood, 84, 3613-36 (1994).
- Glader BE., Diagnosis and treatment of glucose-6-phosphate dehydrogenase deficiency, Hum. Genet., 92, 98-101 (2004).
- Gaetani GF., Galiano S., Canepa L, Ferraris AM. and Kirkman HN., Catalase and glutathione peroxidase are equally active in detoxification of hydrogen peroxide in human erythrocytes, Blood, 73 (1), 334-39 (1989).
- Mehta A., Mason PJ. and Vulliamy TJ., Glucose-6-phosphate dehydrogenase deficiency, Baillieres Best Pract Res Clin Haematol, 13, 21–38 (2000).
- Beutler E., G6PD: population genetics and clinical manifestations, Blood Rev., 10 (1), 45-52 (1996).
- Tishkoff SA., Varcony R. and Chainhinan N. and et al., Haplotype diversity and linkage disequilibrium at human G6PD: Recent origin of alleles that confer malaria resistance, Science, 293 (5529), 455-62 (2001).
- Beutler E., Vulliamy T. and Luzzatto L., Hematologically important mutations: glucose-6-phosphate dehydrogenase, Blood Cells Mol Dis., 22 (4), 49-56 (1996).
- Vulliamy T., Othman A., Town M., Nathwani A., Falusi AG., Mason PJ. and Luzzatto L., Polymorphic sites in African population detected by sequence analysis of the glucose-6-phosphate dehydrogenase gene outline the evolution of the variants A and A–, Proc Natl Acad Sci USA., 88 (19), 8568-71 (1991).
- Beutler, E. and Kuhl W., The NT 1311 polymorphism of G6PD: G6PD Mediterranean mutation may have originated independently in Europe and Asia, Am. J. Hum. Genet, 47 (6), 1008-1012 (1990).
- Mesbah-Nammin SA., Sanati MH., Mowjoodi A, Mason PJ., Vulliamy TJ. and Noori-Daloii MR., Three major glucose-6-phosphate dehydrogenase-deficient polymorphic variants identified in Mazandaran state of Iran, British J. Haematol., 117 (3), 763-64 (2002).
- Noori-Daloii MR., Soltanian S., Mohammad Gangi SH., Yousefi A., Hejazi S., Bani-Hashem A., Hitratfar S. and Sanati M., Molecular identification of the most prevalent mutations of g6pd gene in deficient patients in Khorasan province of Iran, J. Sci. IR Iran, 17(2), 103-106 (2006).
- WHO Working Group: World map of G6PD deficiency, Bull WHO, 67, 601-611 (1989).
- Aksoy M., Dincol G. and Erdem S., Survey on Haemoglobin Variants, β-Thalassaemia, Glucose-6-Phosphate Dehydrogenase Deficiency and Haptoglobin Types in Turkish People Living in Manavgat, Serik and Boztepe (Antalya), Hum. Hered., 30, 3-6 (1980).
- McCurdy PR and Mahmood L., Red cell G6PD deficiency in Pakistan, J. Lab. Clin. Med., 76, 943-948 (1970).
- Ayene I., Glucose-6-phosphate dehydrogenase deficiency in India, Indian J. pediatr, 71, 525-29 (2004).
- Dash S., Hemoglobinopathies, G6PD deficiency, and hereditary elliptocytosis in Bahrain, Hum. Biol., 76 (5), 779-83 (2004).
- Alfadahli S., Kaaba S., Elshafey A., Salim M., Alawadi A. and Bastaki L., Molecular characterization of glucose-6-phosphate dehydrogenase gene defects in the Kuwaiti population, Arch Pathol. Lab. Med. 129 (9), 1144-47 (2005).
- Daar S., Vulliamy TJ. and Kaeda J., Molecular characterization of glucose-6-phosphate dehydrogenase deficiency in Oman, Hum. Hered. 46(3), 172-76 (1996).
- Hilmi FA., Al-Allawi M., Rassam M., Al-Shamma G. and Al-Hashimi A., Red cell glucose-6-phosphate dehydrogenase phenotype in Iraq, East Mediterr. Health. J. 8 (1), 42-48 (2002).
- Al-Ali AK., Al-Mustafa ZH., Al-Madan M., Qaw F. and Al-Ateeq S., Molecular characterization of glucose-6-phosphate dehydrogenase deficiency in eastern province of Saudi Arabia, Clin Chem Lab Med., 40 (8), 814-6 (2002).
- Kazemi Nezhad SR., Mashayekhi A., Khatami SR., Daneshmand S. and et al., Prevalence and molecular identification of Mediterranean glucose-6-phosphate dehydrogenase deficiency in Khuzestan province, Iran, Iranian J. Publ. Health, 38 (3), 127-131 (2009).
- Rahimi Z., Vaisi-Raygani A., Nagel RL. and Muniz A., Molecular characterization of glucose-6-phosphate dehydrogenase deficiency in the Kurdish population of Western Iran, Blood Cells Mol. Dis. 37 (2), 91-94 (2006).
- Kurdi-Haidar B., Mason PJ., Berrebi A., et al., Origin and spread of the glucose-6-phosphate dehydrogenase variant (G6PD-Mediterranean) in the Middle East, Am. J. Hum. Genet., 47 (6), 1013-19 (1990).
- Sukumar S., Mukherjee M.B., Colah R.B. and Mohanty D., Molecular basis of G6PD deficiency in India, Blood Cells Mol. Dis., 33, 141-145 (2004).
- Samilchuk E., Al-Suliman I., Usanga E. and Al Awadi S., Glucose-6-phosphate dehydrogenase (G6PD) mutations and UDP-glucuronosyltransferase promoter polymorphism among G6PD deficient Kuwaitis, Blood Cells Mol. Dis., 31 (2), 201-205 (2003).

This work is licensed under a Creative Commons Attribution 4.0 International License.





