Manuscript accepted on : July 20, 2009
Published online on: 28-12-2009
C. Arunachalam1* and R. Rajasekaran2
1PG and Research Department of Microbiology, Sri Venkateshwara College of Arts and Science, Peravurani, Thanjavur (Dt), India
2PG and Research Department of Botany and Microbiology, A Veeriya Vandaiyar Memorial Sri pushpam College (Autonomous), Poondi, Thanjavur - 613 503 India.
Corresponding Author E-mail: agro_arun@rediffmail.com
ABSTRACT: Pseudomonas aeruginosa is a bacterium responsible for causing life threatening infections like nosocomical infections, life threatening infections in immune compromised persons. The bacterium’s virulence depends on the numerical abundance of cells associated with extracellular factors. The study was taken up to isolate and to understand the biochemical characterization of P. aeruginosa isolated from pus sample (S1), urine samples (S2) Sputum sample (S3) and soil sample (S4). The multiple drug resistance property of P. aeruginosa was clearly demonstrated by Kirby-Bauer’s disc diffusion method. Pseudomonas aeruginosa (S1) isolated from pus sample was selected for pigment studies, since this strain showed good resistance to various antibiotics than others. Pyocyanin (1-hydroxy-N-Methyl phenazine) is a bluish green, chloroform soluble, cytotoxic pigment secreted by the bacterial species of P. aeruginosa. Tech agar was used for the production of pyocyanin pigment and the pigmented broth tube was centrifuged and the pigment was extracted using choloroform. The extracted pigment showed antibacterial activity against E.coli, Proteus vulgaris and Staphylococcus aureus.
KEYWORDS: Clinical samples; Pseudomonas aeruginosa; Pyocyanin; and Antibacterial activity
Download this article as:| Copy the following to cite this article: Arunachalam. C, Rajasekaran. R. PStudies on Antibiotic Resistance of Pseudomonas Aeruginosa Isolated From Various Sample With Special Reference to the Antibacterial Activity of its Pyocyanin Pigment. Biosci Biotechnol Res Asia 2009;6(2) |
| Copy the following to cite this URL: Arunachalam. C, Rajasekaran. R. PStudies on Antibiotic Resistance of Pseudomonas Aeruginosa Isolated From Various Sample With Special Reference to the Antibacterial Activity of its Pyocyanin Pigment. Biosci Biotechnol Res Asia 2009;6(2). Available from: https://www.biotech-asia.org/?p=8939. |
Introduction
The members of Pseudomonas are strictly aerobic, catalase positive, oxidase positive, Gram negative motile bacilli. Their metabolism is respiratory but never fermentative. An RNA group 1 comprises the fluorescens group including P. aeruginosa, P. fluorescens and P. putida1. Pseudomonas aeruginosa can occur as 5 distinct types ranging from dwarf colonies to a large mucoid type2. Three types of bacteriocins (pyocins) are produced by P. aeruginosa which are known as R, F and S3.
Pseudomonas aeruginosa produces a variety of enzymes and toxins4. Besides this, remarkable number of bacterial factors have been postulated playing a role in P. aeruginosa infections5. Further, P. aeruginosa secretes a number of virulence factors, including elastases, rhamnolipids, alginate, exotoxin A, exoenzyme S, and phospholipase C, whose virulent properties have been elucidated well6, 7. Two in vivo murine respiratory tract infection models, acute and chronic, have been demonstrated as the absolute necessity of pyocyanin biosynthesis for P. aeruginosa virulence 8.
Pseudomonas aeruginosa is a bacterium responsible for severe nosocomial infections, life-threatening infections in immuno- compromised persons. The bacterium’s virulence depends on a large number of cells-associated with extracellular factors9. Chronic Pseudomonas aeruginosa infections occur frequently with the immunocompromised, the aged, and those with bronchiectasis10, and otitis externa11.
Pyocyanin (1-hydroxy-N-methylphenazine) is a cytotoxic pigment secreted by the bacterial species Pseudomonas aeruginosa, which frequently infects the lungs of immunosuppressed patients. Pyocyanin toxicity results presumably from the ability of the compound to undergo reduction by NAD (P)H and subsequent generation of superoxide and H2O2 directly in the lungs12. Pyocyanin triggers tissue damage mainly by its redox cycling and induction of reactive oxygen species13. Anti – Staphylococcal activity by Pseudomonas aeruginosa was investigated through the use of the reverse agar plate and the filter paper stamp methods14.
Materials and Methods
Sample collection and Isolation
Different types of clinical samples such as pus samples, urine samples, and sputum samples were collected from hospitalized patients at Meenakshi Mission Hospital and Research centre, Madurai. Pseudomonas aeruginosa was isolated from soil by serial dilution technique the samples were streaked on blood agar and Macconkey agar. The selected isolates were characterized by different biochemical tests. Antibiotic sensitivity test was done by Kirby-Bauer’s disc diffusion method.
Production of pigment and extration
Pseudomonas-Agar P promotes pyocyanin production15. Pseudomonas-Agar P was prepared for the production of pyocyanin. The plates inoculated with isolated strains and incubated at 35 + 2oC for 24 hours. If the isolate fails to grow or grow slowly, the isolate may be re incubate at 30oC for 1-2 days and observe for growth and pigment production. The presence of pyocyanin may be confirmed by adding several drops of chloroform and observe for a blue colour in the chloroform 16.
Extraction of non polar compounds from crude P. aeruginosa culture supernatants was performed by adsorption to an octadecylsilance bonded phase chromatographic material. After thorough washing of sep-pak cartridges with Sorenson buffer, pH 7.6, 20ml of bacterial culture supernatants diluted 1:1 in buffer were passed through the cartridges. After additional washing with buffer, sequential elution with 5ml of chloroform and 5ml of methanol were performed17.
Results and Discussion
Psedomonas aeruginosa was isolated from pus samples, urine samples, sputum samples and soil sample. S1 – isolate from pus sample; S2 – isolate from urine sample; S3 – isolate from sputum sample and S4 – isolate from soil sample were selected. They were confirmed by morphological, bacteriological and biochemical characterization(Table-1). Pseudomonas aeruginosa colonies have a spreading habit, and give a characteristic grape like fruity odour due to the production of aminoacetophenone. Bio chemical reaction on TSI show alkali / alkali (slant / butt) – typical of non fermenters, H2S negative18.
Table 1: Biochemical characterization of Pseudomonas aeruginosa.
| Biochemical test | Pathogenic strains from clinical sample | Strain from soil sample | ||
| S1 | S2 | S3 | S4 | |
| Indole | – | – | – | – |
| TSI agar | – | – | – | – |
| Citrate | + | + | + | + |
| Urease | + | + | + | + |
| Mannitol fermentation | – | – | – | – |
| Mannitol motility | + | + | + | + |
| Catalase | + | + | + | + |
| Oxidase | + | + | + | + |
The multiple drug resistance property of pseudomonas aeruginosa was clearly demonstrated by Kirby-Bauer’s disc diffusion method. Both the clinical and soil isolates showed antibiotics resistance to ampicillin, cefazolin, cofactor, cefdinir and Nolidixic acid. But P. aeruginosa sensitive to amikacin, cefatazidime, gentamycin and cefoperazone as evident by the inhibition zone, prove that the drug will be clinically effective (Table-2). Pseudomonas agar plates were used for production of pyocyanin pigment. The antibacterial activity of pyocyanin pigment of P.aeruginosa was analysed. The extracted pigment showed antibacterial activity against Escherichia coli, Proteus vulgaris, Staphylococcus aureus. (Table – 3, Fig 1-3). Pyocyanin is a cytotoxic pigment secreted by the bacterial species P. aeruginosa. Approximately 50% of clinical Pseudomonas aeruginosa isolates were found to produce pyocyanin at 37oC. Finally conclude that the pigment of Pseudomonas aeruginosa showed good antibacterial activity against selected bacterial culture. Among this, Proteus vulgaris strain highly sensitive to pyocyanin.
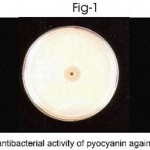 |
Figure 1
|
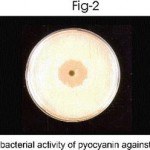 |
Figure 2
|
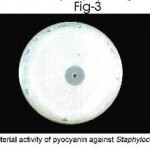 |
Figure 3
|
Table 2: Antibiotic susceptibility test (Disc Diffusion Method).
| S.
No |
Antibiotics | Strain isolated from pus (S1) | Strain isolated from urine (S2) | Strain isolated from Sputum (S3) | Strain isolated from Soil (S4) | ||||
| Zone diameter
(mm) |
Sensitivity
|
Zone diameter (mm) | Sensitivity | Zone Diameter
(mm) |
Sensitivity | Zone diameter (mm) | Sensitivity | ||
| 1 | Ampicillin | – | R | – | R | – | R | – | R |
| 2 | Amoxycillin +
Clavulanic acid |
– | R | 14mm | S | – | R | – | R |
| 3 | Amikacin | 14mm | S | 20mm | S | 23mm | S | 23mm | S |
| 4 | Cefdinir | – | R | – | R | – | R | – | R |
| 5 | Cefazolin | – | R | – | R | – | R | 8mm | R |
| 6 | Ceftazidime | 20mm | S | 10mm | R | 20mm | S | 35mm | S |
| 7 | Co-Trimoxazole | – | R | – | R | 10mm | R | – | R |
| 8 | Cefaclor | – | R | – | R | – | R | – | R |
| 9 | Gentamicin | – | R | 21mm | S | 17mm | S | 18mm | S |
| 10 | Ceficime | – | R | 9mm | R | 8mm | R | 13mm | S |
| 11 | Nalidixic acid | – | R | – | R | 7mm | R | – | R |
| 12 | Cefoperazone | 20mm | S | 13mm | S | 20mm | S | 29mm | S |
(-) No zone, (R) Resistant, (S) Sensitive
Table 3: Antibacterial activity of pyocyanin pigment against few bacterial strains.
| Pigments | Diameter of zone of inhibition in mm | ||
| Escherichia coli | Proteus vulgaris | Staphylococcus aureus | |
| Pyocyanin | 14 | 19 | 15 |
Acknowledgements
I wish to sincerely record my deepest gratitude to Dr. V. Ramaiyan M.Sc., Ph.D., Research advisor, Sri Venkateshwara College of Arts and Science, Peravurani for their valuable and enthusiastic encouragement at very state of this work.
References
- Gilardi G.L., Annals of internal medicine. 77, 211. (1972).
- Phillips I., Journal of medical microbiology. 2, 9(1969).
- Govan J.R.W., Norris and Bergan London: Academic press. 59(1978).
- Young L.S and Armstrong D., Review in clinical Laboratory Science. 3: 291(1972).
- Gallway D.R., J. Molecular microbiology. 51, 2315-2321(1991).
- Lyczak J.B., Cannon C.L., Pier G.B., Microbes infect 2: 1051-1060(2000).
- Lau G.W., Hassetl D.J., Ran H and Kong F., Trends Mol Med 10: 599-606(2004).
- Lau G.W., Ran H., Kong F and Marrodi D., 72: 4375-4278(2004).
- Christian van Delden and Iglewski B.W., Emerging infectious diseases. 4 (4) (1988).
- Khalid M., Saleemi S., Zeitouni M., Ann Saudi Med 24: 284-287 (200).
- Beers, S.L., Abramo, T.J. Review pediatr emerg care 20: 250-256(2004).
- Reszka K.J., O’Malley Y.Q and Britigan B.E., Free Radical Biology and Medicine. 36(11): 1448-1459 (2004).
- Rajkumar cheluvappa, Ronald shimmon and Hilmer S.N., Acta Biochimica polonica 55(3): 571-580(2008)
- Ogino J., Yamada, T., J. Hospital infection. 57(2): 170-174 (2004).
- King, ward and Raney, J. Lab. Clin. Microbiol 44:301 (1954).
- United States pharmacopeial convention, Inc. Rockville, Md. (2001).
- Sorensen R.U., Klinger J.D., Cash H.A and Dearborn D.G., Infection and immunity. 41(1): 321-330(1983).
- Mukerjee K.L., Medical Laboratory Technology. 2: 571-573,612 (1988).

This work is licensed under a Creative Commons Attribution 4.0 International License.





