Manuscript accepted on : March 10, 2009
Published online on: 28-06-2009
R. Sharada1, K. Swathi2, S. Venkateshwar2, M. Ananda Rao1 and M. Narsi Reddy2
1Department of Chemistry,University College of Science, Osmania University, Hyderabad India.
2Centre for Biotechnology,University College of Technology, Osmania University, Hyderabad India.
Corresponding Author E-mail: mnreddydr@gmail.com
ABSTRACT: Cellulases are complex enzymes and are capable of degrading lignocellulosic materials. These enzymes are produced by the fungus and bacteria. This work mainly focus on factors relevant for improvement of enzyme hydrolysis by using Aspergillus niger (MTCC2196) in submerged fermentation. Several cultural conditions were examined to access their effect in optimizing enzyme production. The Aspergillus niger (MTCC2196) could grow upto 28±2°C within a broad pH range of 3.0 to 6.0 with an optimal growth temperature and pH at 28°C and 5.0 respectively. For the Aspergillus niger (MTCC2196), cellulase production occurred between temperature 25°C to 30°C and pH 3.0 to 8.0. Maximum growth and enzyme production was observed at eighth day, when grown in 50ml of medium at pH 5.0, inoculum level 2ml and nitrogen sources like peptone 5mg, beef extract 5mg were added under shaking conditions at 280C. The results showed that Aspergillus niger (MTCC2196) under study is a good producer of cellulases.
KEYWORDS:
Cellulase; Aspergillus niger (MTCC2196); submerged fermentation; lignocellulosic material
Download this article as:| Copy the following to cite this article: Sharada R, Swathi K, Venkateshwar S, Rao M. A, Reddy M. N. Optimization of Cultural Conditions for Cellulase Production by Aspergillus Niger (MTCC2196) Using Submerged Fermentation. Biosci Biotechnol Res Asia 2009;6(1) |
| Copy the following to cite this URL: Sharada R, Swathi K, Venkateshwar S, Rao M. A, Reddy M. N. Optimization of Cultural Conditions for Cellulase Production by Aspergillus Niger (MTCC2196) Using Submerged Fermentation. Biosci Biotechnol Res Asia 2009;6(1). Available from: https://www.biotech-asia.org/?p=8185 |
Introduction
Cellulose, being an abundant and renewable resource, is a potential raw material for the microbial production of food, fuel and chemicals (Coughlan, 1985). Various bacteria, actinomycetes and filamentous fungi produce extracellular cellulases (Pothiraj,C., et al., 2006).
Cellulases are a complex enzyme system, comprising of endo-1,4-β-D-glucanase (EC-3.2.1.4), exo-1,4-β-glucanase (exo-cellobiohydrolase, EC-3.2.1.91) and β-D-glucosidase (β-D-glucoside glucanhydrolase, EC-3.2.1.21). They together with other related enzymes namely hemicellulases and pectinases, are among most important group of enzymes which are employed in the processing of lignocellulosic materials for the production of feed, fuel and chemical feed stock (Ashok Pandey et al., 2006).
Cellulases have a wide range of applications. Potential applications are in food, animal feed, textile, fuel, chemical industries, paper and pulp industry, waste management, medical/pharmaceutical industry, protoplast production, genetic engineering and pollution /effluent treatment (Tarek,A.A. et al., 2007; Beguin et al., 1993; Mandels, 1985; Coughlan, 1985).
The objective of this investigation is to study the production of cellulases by Aspergillus niger MTCC 2196 obtained from MTCC of IMTECH, Chandigarh, as well as the influence of different cultural conditions on enzyme production by this species in the laboratory. This organism is known as an excellent producer of cellulolytic enzymes (Tarek,A.A. et al., 2007; El-Abyad et al., 1996, 1997; Kurosawa et al., 1989; Lachke et al., 1988; Moussa,1994).
Materials and Methods
Chemicals
All chemicals and medium constituents used for the present study were procured from M/s Hi-media, Bombay (India).
Microorganism
Aspergillus niger MTCC2196 obtained from Culture collection of microbial type culture collection of IMTECH, Chandigarh, and was maintained on PDA slants at 40C. Then it is subcultured once in a month. Spores were harvested from a 9 day old PDA slant in 5 ml of sterile distilled water.
Culture conditions
The organism was cultured by submerged fermentation in modified potato dextrose broth (MPD broth). Medium contains the following ingredients (g / 100 ml): Potato infusion, 20; Dextrose, 2.0; K2HPO4, 0.1; MgSO4, 0.05; FeSO4, 0.001 and NaNO3, 0.3. Four sets of 100 ml each of MPD broth at pH 5.0 was inoculated by Aspergillus niger MTCC 2196. These were kept on a shaker at 120-150 rpm and 28±20C for 8 days before harvesting. The fungal mycelia were harvested by centrifugation (REMI C24) at 5000 rpm for 20 minutes. The pellet was homogenized at 6000 rpm using acetate buffer (pH 4.8) and centrifuged subsequently at 6000 rpm for 30 minutes to extract the total fungal protein. The temperature during the course of extraction was maintained at 40C. The supernatant was used as the crude enzyme preparation.
Effect of pH
The effect of pH was studied by adjusting the pH ranged from 3.0 to 8.0 of fermentation media. The pH of the medium was adjusted by using 1N HCl or 1N NaOH. After inoculating 2ml of spore solution of Aspergillus niger MTCC 2196, the flasks were kept in orbital shaker incubator (Lab Tech LSI-3016A2) at 28±20C at 120-150 rpm.
Effect of temperature
Effect of temperature was determined by incubating fermentation medium at 250C to 300C in orbital shaker incubator (Lab Tech LSI-3016A2) at 120-150 rpm. After regular intervals, enzyme assay was performed.
Effect of inoculum level
Fermentation media inoculated with 1 ml to 5 ml of spore solution of Aspergillus niger MTCC 2196. After inoculation the flasks were incubated in orbital shaker incubator (Lab Tech LSI-3016A2) at 120-150 rpm. At regular intervals, enzyme assay was performed.
Effect of nitrogen source
For optimization, different nitrogen sources used were peptone and beef extract. These sources were used in different concentrations. Peptone was used in range from (mg/10ml) 1to 5 .The beef extract was used in range from (mg / 10 ml) 1 to 5 in fermentation medium. All the flasks were incubated at 28±20C in orbital shaker incubator (Lab Tech LSI-3016A2) at 120-150 rpm. At regular intervals enzyme assay was performed.
Enzyme assay
The cellulase estimation was done by the method of Mendel’s and Weber (1969).50 mg of Whatman No.1 filter paper strips were incubated with 1 ml acetate buffer (pH 4.8) and 0.5 ml of undiluted sample of the extracted enzyme at 500C for 1 hour. The reducing sugars released were estimated by the dinitrosalicylic acid (DNSA) reagent (Miller, 1959). The cellulase enzyme activity was expressed in terms of milligrams of reducing sugars released per milliliter of the enzyme extract.
Protein estimation
The protein was measured in the culture supernatant, and estimated by Modified Lowry’s method (1951).
Results and Discussion
The Aspergillus niger MTCC 2196 was selected for the production of cellulases in submerged fermentation since the Aspergillus niger MTCC 2196 produced cellulase over the entire range of pH (3.0 – 8.0). However, maximum cellulase production was observed at pH 5.0. While at pH 5.5 the cellulase production was about 70% (Fig.1). Most commercially available cellulases have been reported from the genus Aspergillus (Narasimha,G.,et al.,2006).
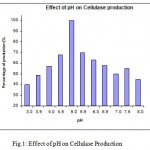 |
Figure 1: Effect of pH on Cellulase Production.
|
Based on the exhibited cellulase activity and growth temperature range of 25 – 300C, and the effect of temperature on cellulase production was determined. The Aspergillus niger MTCC 2196 exhibited maximum cellulase activity at 280C. While, at temperature 28.50C the cellulase production was about 75% (Fig.2).
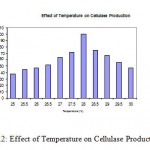 |
Figure 2: Effect of Temperature on Cellulase Production.
|
The results in Fig.3 showed the effect of inoculum level on cellulase production. The flasks were inoculated with 1ml to 5ml of spore solution, where the maximum cellulase production was observed at 2 ml inoculum level. While at 3ml inoculum level the cellulase activity was about 80%.
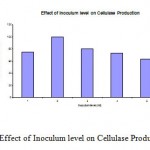 |
Figure 3: Effect of Inoculum level on Cellulase Production.
|
Among the various nitrogen sources tested for the cellulase production, peptone proved to be the best single nitrogen source for the enzyme production followed by the beef extract, yeast extract etc. (Fig.4).
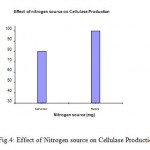 |
Figure 4: Effect of Nitrogen source on Cellulase Production.
|
Acknowledgement
The author wish to thank research scholars J.Swarnalatha, Y. Neeraja & M.Anamika at Centre for Biotechnology, University College of Technology, Osmania University, Hyderabad, for their friendly co-operation.
References
- Ashok Pandey, Selvakumar, P., Carlos, R. Soccol ., and Poonam Nigam. (2006). Solid state fermentation for the production of industrial enzymes. pp.1-23
- Beguin, P., Anbert, J.P. (1993). The biological degradation of cellulose. FEMS microbial. Rev. 13: 25-58
- Coughlan, M.P. ( 1985). Cellulase: Production properties and applications. Biochem.Soc.Trans. 13: 405-406.
- El-Abyad , M.S., Abu-Taleb, A.M., Abdel-Mawgoud,T. (1996). Effect of the herbicide pyradue on host cell wall degradation by the sugarbeet pathogens Rhizoctona solani and Sclerotium rolfsii. Can.J. Bot. 74: 1407-1415.
- El-Abyad , M.S., Abu-Taleb, A.M., Abdel-Mawgoud,T. (1997). Response of host cultivar to cell wall degrading enzymes of the sugarbeet pathogens Rhizoctona solani khun solani and Sclerotium rolfsii Sacc. under salinity stress. Microbiol. Res. 152: 9-17.
- Kurosawa, K., Hosoguchi, M., Hariantono, J., Sasaki,H., Takao, S. (1989). Degradation of tough materials by cellulase from Corticium Rolfsii. Agric. Boil. Chem. 53: 931-937.
- Lachke, A.H., Despande, M.V. (1988). Sclerotium rolfsii: status in cellulase research. FEMS Microbiol. Rev. 54: 177-194.
- Lowry, O.H., Rosenbrough, N.J., Farr, A.I., and Randall, R.J. (1951). Protein measurement with the folin-phenol reagent. J. Biol. Chem. 193, 265-275.
- Mandels, M. ( 1985). Applications of cellulases. Biochem.Soc.Trans. 13: 414-415.
- Mendels, M., and Weber, J. (1969). The production of cellulase. Adv. Chem.Series 95, 391-414.
- Miller, G.L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal. Chem, 31, 426-428.
- Moussa, T.T.A. (1994). Preliminary studies on the production of pathogenicity enzymes by some sugarbeet pathogenic fungi under the stresses of salinity and herbicidal treatment. M.Sc Thesis, Depatment, Botany. Fac. Sci. Univ.Cairo, pp. 1-132.
- Narasimha, G., sridevi, A., Buddolla, V., Subhosh,C.M.,and Rajsekhar, R.B.(2006). Nutrient effect on production of cellulolytic enzymes byAspergillus niger. Afr. J Biotechnol. 5(5): 472-476.
- Pothiraj,C., Balaji,P., and Eyini,M. (2006). Enhanced production of cellulases by various fungal cultures in solid state fermentation of cassava waste. African Journal of Biotechnology Vol.5(20), pp. 1882-1885.
- Tarek,A.A.Moussa., and Nagwa, A. Tharwat. (2007). Optimization of cellulase and β- glucosidase induction by sugarbeet pathogen Sclerotium rolfsii. African Journal of Biotechnology. Vol.6 (8), pp. 1048-1054.

This work is licensed under a Creative Commons Attribution 4.0 International License.





