Manuscript accepted on : December 04, 2008
Published online on: 11-03-2016
Abdellah Akhkha
Biology Department, Faculty of Science, Taibah University, Madinah Munawwarah, (Kingdom of Saudi Arabia).
ABSTRACT: The compensatory photosynthesis in uninfected fourth leaf of infected plants was investigated in cv. Prisma and the two wild lines B19909 and I-17-40. Infection of the three lower leaves increased the rate of photosynthesis as well as the quantum efficiency in the powdery mildew tolerant line B19909 only. This indicates that compensatory photosynthesis may play an important role in tolerance of the parasite.
KEYWORDS: Blumeria graminis; compensatory photosynthesi; wild barley lines
Download this article as:| Copy the following to cite this article: Akhkha A. The Effect of Powdery Mildew (Blumeria Graminis F. Sp. Hordei) on Compensatory Photosynthesis and Dark Respiration in the Uninfected Fourth Leaf of Infected Cultivated and Wild Barley Lines. Biosci Biotechnol Res Asia 2008;5(2) |
| Copy the following to cite this URL: Akhkha A. The Effect of Powdery Mildew (Blumeria Graminis F. Sp. Hordei) on Compensatory Photosynthesis and Dark Respiration in the Uninfected Fourth Leaf of Infected Cultivated and Wild Barley Lines. Biosci Biotechnol Res Asia 2008;5(2). Available from: https://www.biotech-asia.org/?p=7121 |
Introduction
Water plays a very important role in all physiological processes in plants including photosynthesis, respiration, translocation, partitioning of metabolites, stomatal behaviour, protein synthesis, cell division, cell elongation and cell wall synthesis. Water stress will lead to the perturbation of all or some of these physiological processes and consequently will lead to reductions in plant growth and yield (Kramer, 1983).
Healthy plants can protect themselves against the development of water stress by regulating stomatal aperture. The stomata are sensitive structures that represent the greatest variable resistance in the pathway of water movement through the plant (Ayres, 1981) and any biotic or abiotic factor causing changes in the pattern of stomatal behaviour will affect plant water relations and consequently perturb growth and development. Many investigations have been carried out to determine the effects of obligate biotrophs on stomatal behaviour. These effects have been found to differ from one pathogen to another and from one host to another.
Infections of barley leaves by Rhynchosporium secalis (Ayres et al., 1975) and of potato by the blight fungus, Phytophthora infestans (Farrell et al., 1969) caused an increase in the rate of transpiration from the infected area of the leaf both in the light and in the dark. This increase was attributed to an increase in the mean stomatal aperture in the infected area in the light and the failure of the stomata to close in the dark. The downy mildew fungus Peronospora tabacina has also been found to affect stomatal opening in the leaves of its host tobacco in a similar manner (Cruickshank et al., 1961).
In contrast, stomatal opening in the light has been reported to be inhibited by rust and powdery mildew infections as well as by some viruses such as sugar beet yellows virus (Hall et al., 1972).
Transpiration in rust and powdery mildew infected tissues usually follows the pattern of stomatal behaviour, decreasing in the light and increasing in the dark (Walters, 1985). Rust fungi enter their hosts through stomatal pores, develop mainly in the intercellular spaces of the leaf and inhibit stomatal movements progressively until eventually the stomata became fixed in an almost closed position (Duniway et al., 1971). However, with rust fungi, once fungal sporulation has ruptured the cuticle, non-stomatal transpiration increases and becomes the significant factor (Johnson et al., 1934, 1940 and Murphy, 1935).
Paul et al. (1984) showed that after sporulation, groundsel (Senecio vulgaris) leaves infected with Puccinia lagenophorae transpired much more rapidly than did healthy controls. The same results were shown by Duniway et al. (1971a) in bean (Phaseolus vulgaris) leaves infected with Uromyces phaseolus.
Powdery mildew infections result generally in a failure of stomata to open fully in the light and to close fully in the dark (Majernick, 1971; Ayres, 1976). Wheat leaves infected with B. graminis f.sp. tritici were shown to have a significantly reduced stomatal opening within three to six hours after inoculation (Martin et al., 1975). Majernick (1965) working with barley leaves infected with B. graminis f.sp. hordei reported that stomatal transpiration had reduced within one day after inoculation. Ayres (1979) also, using barley leaves infected with B. graminis f.sp. hordei, observed that reduced stomatal opening was not apparent until three days after inoculation. Other plant species other than cereals showed similar responses to mildew infection. For example, although garden pea (Pisum sativum) leaves infected with E. pisi showed an initial increase in stomatal opening within the first 48 hours of inoculation, the stomatal opening became progressively reduced in the light and stomata failed to close completely in the dark (Ayres, 1976). Thomas et al. (1982) observed a 50% reduction in stomatal aperture five days after inoculation in sugar beet (Beta vulgaris) leaves infected with E. polygoni. Similar responses were observed in leaves of oak plants infected with Microsphaera alphitoides, but not until six days after inoculation, although, transpiration rates increased within two to three days after inoculation (Hewitt et al., 1975).
Ayres (1972 and 1975) investigated stomatal functioning in barley leaves infected with Rhynchosporium secalis and suggested that in the early stages of infection the increase in stomatal aperture was a result of the loss of osmotically active solutes from the epidermal cells of diseased leaves which consequently altered the turgor relations between guard cells and their surrounding epidermal cells. The increase in transpiration at later stages of infection was attributed to water loss through the ruptured cuticle (Ayres, 1975).
In the case of barley infected with B. graminis f.sp. hordei, Majernick (1965) suggested that a volatile product was involved in the inhibition of stomatal opening in the light. A similar suggestion was made by Martin et al. (1975) for wheat leaves infected with B. graminis f.sp. tritici.
The increased stomatal opening in the light that occurs in pea leaves infected with E. pisi 48 hours after inoculation contrasts with the reduced stomatal opening in wheat within 6 hours of inoculation (Martin et al., 1975) and in barley within 24 hours after inoculation (Majernick, 1965) with the cereal powdery mildew. The difference between peas and cereals (barley and wheat) was attributed to the lack of production of a volatile substances in peas infected with Erysiphe pisi or to the differences in the turgor pressures of guard cells and epidermal cells (Ayres, 1976). Furthermore, Ayres (1980) suggested that stomatal opening could be inhibited by substances synthesised by the host such as pisatin (a pterocarpan) which accumulates in pea leaves infected with E. pisi.
The increased rate of transpiration observed in barley leaves infected with B. graminis f.sp. hordei when 50% of the leaf was covered by mildew, was attributed to cuticular injuries caused by the infection (Paulech et al., 1970; Majernick, 1965). In contrast, the increase in the rate of transpiration observed in oak leaves infected with Microsphaera alphitoides was attributed mainly to the fungal mycelium itself (Hewitt et al., 1975).
Many of the differences in host response to different pathogens are most likely to be mainly due to the different ways the pathogens grow and develop on or within their host’s tissues. However, the experimental differences in host response reported for particular pathogens are also likely to be due to an extent to the experimental procedures used, but are also likely to be due to the fact that different cultivars were used.
One significant factor missing from most of the studies was any measure of the way in which or the rate at which parasite biomass accumulated during the course of the experiments. Even when parasite biomass accumulated to similar extents in the different cultivars used, reactions may be different due to different tolerances of the parasite in the tissues.
The effects of infection on stomatal resistance were determined in the two wild barley lines I-17-40 and B19909, and in the cultivated barley cv. Prisma. The experiment was carried out twice, with similar results being obtained on each occasion. Only the results of the second experiment are presented.
Materials and Methods
Plant Material & Growth
The lines of wild barley (Hordeum spontaneum) and of cultivated barley (Hordeum vulgare) used in this study were obtained either from the John Innes Centre, Norwich Research Park or from the Scottish Crop Research Institute, Invergowrie, Dundee.
Seed germination was carried out following Akhkha et al. (2003a) procedure by treating wild lines seeds with 7 days of chilling temperature at 4°C. Seeds of both wild and cultivated lines were germinated in damp filter paper then sown in peat based potting compost (Levington Horticulture Ltd.).
Before use, the wild lines were inbred as mentioned in Akhkha et al. (2003a).
Stomatal measurements
Stomatal resistance
An automatic diffusion porometer MK3 Delta-T Devices (128, Low Road, Burwell, Cambridge CB5 OEJ, UK) was used to measure stomatal diffusive resistance.
Principle of the measurements
The diffusion porometer measures the approximate rate of diffusion of water vapour through the stomata. Its operation assumes that water vapour diffusion out of a leaf into dry air is regulated by the degree of opening of the stomata (neglecting cuticular transpiration). A small chamber containing a relative humidity sensor is clamped to the leaf. Prior to reading, a small electric diaphragm pump blows a stream of air, dried by passing through silica gel, into the chamber. Water vapour emitted by the transpiring leaf surface causes the relative humidity (RH) within the chamber to rise and the sensor becomes moist. As the sensor becomes moist its conductivity increases and the rate of increase in conductivity to a set value is directly proportional to the rate of outward diffusion of water vapour through the stomata. The difference in temperature between the leaf and the cup is measured by two thermistors, which are built into the leaf clamp.
Porometer calibration
The porometer is supplied with a moulded polypropylene calibration plate with six groups of holes each of known diffusion resistance. A source of water vapour is provided by backing the plate with damp filter paper, which is sealed to the plate with waterproof tape. The sensor head is clipped onto the calibration plate and readings are taken from each set of holes. A calibration graph of plate resistances is plotted against the corresponding counts (Automatic porometer MK3 operating manual,) and this graph is used to convert the counts obtained from the leaf measurements into diffusion resistance values.
Experimental procedure
Fifty seedlings were raised. When two weeks old, the fully expanded third leaves on 25 plants of each line were inoculated in the middle region of the adaxial surface, using a camel hair brush. The tip and the base of the leaf blades were kept free of mildew. The other 25 plants of each line were kept free of mildew by adding 0.05% Benlate solution to the pots at weekly intervals. The inoculated and uninoculated plants were then placed randomly in the growth cabinet. The stomatal resistance measurements were taken from four plants per treatment per line. The first measurements in the light were made 24 hours after inoculation, and then the same plants were placed in a dark room for 24 hours after which porometer measurements were made under green light. Subsequent measurements were made at two-day intervals until 5 sets of measurements had been made.
Stomatal resistances were measured in the middle and tip of both adaxial and abaxial surfaces of infected and uninfected third leaves on each plant line.
Statistical Analysis
The means and standard errors (shown in graphs) were calculated using Excel (Microsoft Office 2000). Analysis of variance was performed using Minitab’s ANOVA and General Linear Model (version 13).
Results
Stomatal resistance was measured in the middle and tip regions of infected and uninfected leaves of the three lines. The results are plotted graphically in Figs 1A and 1B.
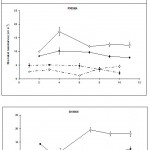 |
Figure 1a
|
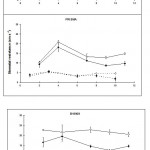 |
Figure 1b
|
Ontogenetic changes in stomatal function in the uninfected third leaf
Changes in the light
Stomatal resistance in the light in both the middle and tip regions of uninfected leaves remained relatively constant throughout the period of measurement, in all three lines (Figs. 1A and 1B).
Changes in the dark
In contrast, in the dark, marked changes occurred as the leaves aged in line I-17-40 and cv. Prisma at both the middle and the tip regions (Figs. 1A and 1B). For cv. Prisma and line I-17-40, at the beginning of the experiment, the stomatal resistances increased reaching a maximum two days later, but from then on, resistances declined and then an approximately steady state was reached and maintained to the end of the experiment.
In line B19909, stomatal resistance increased rapidly in the middle region from the second to the seventh day after inoculation and then stayed at a relatively constant state. In contrast to the other two lines, stomatal resistance in the tips of the leaves of line B19909 remained at an almost constant level during the whole period of the experiment.
Effects of infection on stomatal function in the light
Stomatal resistance in the middle, inoculated region, of the leaf
Within 24 hours of inoculation, stomatal resistance had significantly (p < 0.01) increased in infected leaves when compared to uninfected leaves in all three lines, but then as infection progressed it began to decrease. By the eighth day after inoculation it was similar to that of uninfected leaves in both cv. Prisma and line B19909, but in line I-17-40, it was still slightly higher ten days after inoculation. From the eighth day, the stomatal resistance of cv. Prisma continued to decrease to levels significantly (p < 0.05) below uninfected leaves. In contrast, stomatal resistance in line B19909 remained at control levels.
Stomatal resistance at the leaf tip
Infection in the centre of the leaf increased stomatal resistance in the uninfected tip of that leaf in all three lines (Fig. 1B) although the increase did not become significant, until around 10 days after inoculation (p < 0.05).
Effects of infection on stomatal function in the dark
Stomatal resistance in the middle inoculated region of the leaf
The high stomatal resistances of uninfected leaves in the dark indicate that the stomata were probably closed (Fig. 1A). Infection decreased the stomatal resistance of infected leaves significantly (p < 0.02) in both the wild line I-17-40 and cv. Prisma. As infection progressed the differences became smaller but still remained significant. In contrast, by two days after inoculation, stomatal resistance in leaves of line B19909 had increased significantly above control levels (p < 0.001), but as infection progressed, it decreased again and followed the same pattern as in the other two lines.
The results suggest that infection by powdery mildew prevented the stomata from closing in the dark as fully as those in uninfected leaves.
Stomatal resistance at the leaf tip
Infection had little effect on stomatal resistance in the uninfected tip regions of inoculated leaves of the wild line I-17-40. In contrast, infection decreased stomatal resistance, in uninfected tips of infected leaves, both of cv. Prisma and line B19909. These decreases became significant (p < 0.05) between seven and nine days after inoculation.
Discussion
Measurements of stomatal function in whole leaves of the three lines cv. Prisma and the two wild lines, B19909 and I-17-40 following inoculation showed significant alterations as the result of infection. Stomatal resistance in the light in the inoculated middle region of infected leaves was initially increased by infection in all three lines, but as the leaves became more heavily infected, it began to decrease to a level similar to that of uninfected plants in line B19909 but lower than the uninfected plants in cv. Prisma. In contrast, stomatal resistance in the dark in the inoculated middle regions of infected leaves began to fall in both line I-17-40 and cv. Prisma from two and four days after inoculation respectively, but not until seven days after inoculation in line B19909. However, stomatal resistance eventually fell to lower levels in line B19909 than in the other two lines.
The initial increase in stomatal resistance in infected leaves in the light is likely to reduce the diffusion of CO2 to the mesophyll cells and could thus be partly responsible for the decline in photosynthesis that occurred following inoculation. However, the subsequent reductions in stomatal resistance should allow increased CO2 uptake. Altered stomatal behaviour following infection could also be expected to alter the rate of transpiration and the leaf water content, and reductions in leaf water content could affect rates of photosynthesis.
The transpiration rate from leaves usually follows the pattern of stomatal behaviour. Thus in mildew infected barley leaves transpiration would be expected to initially decrease in the light because infection causes the stomata to close. It would also increase in the dark, when the stomata failed to close completely. Changes in transpiration in infected leaves could also result partly from the increase in the boundary layer resistance caused by the presence of the fungal mycelium over the leaf surface and partly from the mycelium itself which also provides an increased route for cuticular transpiration. In the light, stomatal resistance increased significantly from 24 hours after inoculation when mildew development was very limited in all three lines, suggesting that the stomata provided the main control over water loss during the early stages of infection. However, in the dark, stomatal resistance decreased from 48 hours after inoculation in cv. Prisma, but not until 72 hours after inoculation in line I-17-40 and 168 hours after inoculation in line B19909. Thus stomatal function was impaired at a much earlier stage in cv. Prisma than in leaves of line B19909, the latter being able to control water loss up to quite a late stage of infection when 25% or more of the leaf area was colonised by mildew.
The increased stomatal closure in the light from 24 hours after inoculation of barley leaves is in agreement with the observations of Majernik (1965) who found that B. graminis f.sp. hordei decreased stomatal opening of barley leaves from 24 hours after inoculation. Martin et al. (1975) also observed that mildew infection decreased stomatal opening but slightly earlier than Majernik (1965), from around six hours after inoculation. However, neither of these studies related changes in stomatal function to the amount of mildew present at the time of stomatal resistance change and also they used different cultivars to the ones used here.
Infection also decreased stomatal opening in the light in mildewed pea leaves from three days after inoculation (Ayres, 1976) and from five days after inoculation in mildewed oat plants (Sabri, 1993). However, an initial increase in stomatal aperture was observed in pea leaves 48 hours after inoculation with Erysiphe pisi (Ayres, 1976) and in oat plants 72 hours after inoculation (Sabri, 1993). Martin et al. (1975) and Majernik (1965) in studies of wheat infected with B. graminis f.sp. tritici suggested that a volatile product of the fungus could be involved in the alteration of stomatal behaviour. If this is so, it may be that B. graminis f.sp. hordei produces a similar substance. However, other causes have been suggested, such as infection induced changes in the turgor pressures of the guard cells and of other epidermal cells (Ayres, 1976). Future work should concentrate on investigating the involvement of any metabolic substances in the alteration of stomatal behaviour in infected leaves as this could lead to an explanation of plant tolerance or intolerance of diseases.
References
- Akhkha, A; Clark, D. D. & Dominy, P. J. ( 2003a): Relative tolerances of wild and cultivated barley to infection by Blumeria graminis f.sp. hordei (Syn. Erysiphe graminis f.sp. hordei). I-the effects of infection on growth and development. Physiological & Molecular Plant Pathology 62(4), 237-250.
- Akhkha, A; Clark, D. D. & Dominy, P. J. ( 2003b): Relative tolerances of wild and cultivated barley to infection by Blumeria graminis f.sp. hordei (Syn. Erysiphe graminis f.sp. hordei). II-the effects of infection on photosynthesis and respiration. Physiological & Molecular Plant Pathology 62(6), 347-354.
- Ayres, P. G. & Jones, P. (1975) Increased transpiration and the accumulation of root absorbed 86Rb in barley leaves infected by Rhyncosporium secalis (leaf blotch). Physiological Plant Pathology 7, 49-58.
- Ayres, P. G. (1972) Abnormal behaviour of stomata in barley leaves infected with Rhynchosporium secalis (Oudem) J. J. Davis. Journal of Experimental Botany 23, 683-691.
- Ayres, P. G. (1976) Patterns of stomatal behaviour, transpiration, and CO2 exchange in pea following infection by powdery mildew (Erysiphe graminis f.sp. pisi). Journal of Experimental Botany 27, 354-363.
- Ayres, P. G. (1979) CO2 exchanges in plants infected by obligately biotrophic pathogens. In: Photosynthesis and Plant Development. pp. 343-354. Ed. by Marcelle, R.; Clijsters, H. and Van Poucke, M. Junk, The Hague.
- Ayres, P. G. (1980) Responses of stomata to pathogenic microorganisms. In: Stomatal physiology. Ed. by Jarvis, P. G. & Mansfield, T. A. pp. 205-221. Cambridge University Press. Cambridge.
- Ayres, P. G. (1981) Effects of disease on plant relations. In: Effects of disease on the physiology of growing plant. pp. 131-148. Ed. by Ayres, P. G. Cambridge University Press.
- Cruickshank, I. A. M. & Rider, N. E. (1961) Peronospora tabacina in tobacco: Transpiration, growth and related energy considerations. Australian Journal of Biological Science 14, 45-57.
- Duniway, J. M. & Durbin, R. D. (1971a) Some effects of Uromyces phaseoli on the transpiration rate and stomatal responses of bean leaves. Phytopathology 61, 114-119.
- Farrell, G. M.; Preece, T. F and Wren, M. J. (1969) Effects of infection by Phytophtora infestans (Mont.) De Bary on the stomata of potato leaves. Annals of Applied Biology 63, 265-275.
- Hall, A. E. & Loomis, R. S. (1972) An explanation for the difference in photosynthetic capabilities of healthy and beet yellow virus-infected sugar beets (Beta vulgaris L.). Plant Physiology 50, 576-580.
- Hewitt, H. G. & Ayres, P. G. (1975) Changes in CO2 and water vapour exchange rates in leaves of Quercus robur infected by Microsphaera alphitoides (Powdery mildew). Physiological Plant Pathology 7, 127-137.
- Johnson, C. O. & Miller, E. C. (1934) Relation of leaf-rust infection to yield, growth and water economy of two varieties of wheat. Journal of Agricultural Research 49, 955-981. Cited in Walters (1985).
- Johnson, C. O. & Miller, E. C. (1940) Modification of diurnal transpiration in wheat by infections of Puccinia triticina. Journal of Agricultural Research 61, 427-444. Cited in Walters (1985).
- Kramer, P. J. (1983) Water Deficits and Plant Growth. In: Water Relations of Plants. Ed. by Kramer, P. J. pp. 342-389. Academic Press. London.
- Majernik, O. (1965) Water balance changes of barley infected by Erysiphe graminis D. C. f.sp. hordei Marchal. Phytopathologische Zeitschrift 53, 145-153.
- Majernik, O. (1971) A physiological study of the effects of SO2 pollution, phenylmercuric acetate sprays, and parasites on stomatal behaviour and ageing in barley. Phytopathologische Zeitschrift 72, 255-268.
- Martin, J. F.; Stuckey, R. E.; Safir, G. R. and Ellingboe, A. H. (1975) Reduction of transpiration from wheat caused by germinating conidia of Erysiphe graminis f.sp. tritici. Physiological Plant Pathology 7, 71-77.
- Murphy, H. C. (1935) Effect of crown rust infection on yield and water requirement of oats. Journal of Agricultural Research 50, 387-401. Cited in Walters (1985).
- Paul, N. D. & Ayres, P. G. (1984) Effects of rust and post-infection drought on photosynthesis, growth and water relations in groundsel. Plant Pathology 33, 561-569.
- Paulech, C. & Haspelová – Harvatoviăová, A. (1970) Photosynthesis, plant pigments and transpiration in healthy barley and barley infected with powdery mildew. Biologia, Bratislava. 25, 477-487.
- Sabri, N. (1993) A study of the effects of powdery mildew Erysiphe graminis f.sp. avenae, on the growth and development of wild and cultivated oats. Ph.D. Thesis. Botany Department. University of Glasgow.
- Thomas, R. G. & Duniway, J. M. (1982) Effects of powdery mildew infection on the efficiency of CO2 fixation and light utilisation by sugar beet leaves. Plant Physiology 69, 139-142.
- Walters, D. R. (1985) Shoot : Root interrelationships The effects of obligately biotrophic fungal pathogens. Biological Review 60, 47-79.

This work is licensed under a Creative Commons Attribution 4.0 International License.





