Manuscript accepted on : June 30, 2008
Published online on: 01-03-2016
Synthesis of benzothiazole selenato complexes and determination of its anti-tumor activity
B. Meenakshi2*, M. Bansal1, Shiv K. Gupta1 and Amit K. Das2
1R.V. Northland Institute, 18 Km on Ghaziabad-Bulandshahar G.T. Road, Dadri (Greater Noida Phase II), G. B. Nagar India.
2Krupanidhi College of Pharmacy, Koramangala, Bangaluru India.
Corresponding Author E-mail: mayank_pharma@rediffmail.com
ABSTRACT: Different selenium complexes were synthesized using benzothaizole selenato ligand of Zn (II), Hg (II), Cd (II), Cu (II), and Ni (II). The derivatives M [Se (BTA)]2 (where M=Zn, Ni, Cu, Cd, Hg and BTA- benzothiazole) were prepared by the reaction of MCL2 with lithium arene selenato ligand of BTASe+Li. The compounds synthesised were identified and characterized by various method like melting point, thin layer chromatography, nuclear magnetic resonance, mass spectroscopy, atomic absorption spectroscopy, and screening for anti tumor activity was carried out of all the derivatives using Ehlirch asicites carcinoma (EAC) cells induced in albino mice. Out of those synthesized selenato metallic complexes Zn[Se(BTA)]2, Ni[Se(BTA)]2 were found to be potent anti -tumor since these compounds have shown increase in RBC, decrease in WBC, increase in life span, decrease in ascities fluid volume , increase in lymphocytes significantly.
KEYWORDS: Selenium complexes; Benzothiazole; Ehlirch asicites carcinoma (EAC)
Download this article as:| Copy the following to cite this article: Meenakshi B, Bansal M, Gupta S. K, Das A. K, Synthesis of benzothiazole selenato complexes and determination of its anti-tumor activity. Biosci Biotechnol Res Asia 2008;5(1) |
| Copy the following to cite this URL: Meenakshi B, Bansal M, Gupta S. K, Das A. K, Synthesis of benzothiazole selenato complexes and determination of its anti-tumor activity. Biosci Biotechnol Res Asia 2008;5(1). Available from: https://www.biotech-asia.org/?p=6837 |
Introduction
In last few years there has been considerable interest in the synthesis and characterization of metal complexes of ligands derived from heterocyclic molecule because of their biological significance1. The metal complexes especially of Pd (II), Pt (IV) with various heterocyclic compounds as ligands have also been studied by researches due to their anti-tumor, anti- viral, anti- bacterial, anti -fungal activity2. Various metal complexes of heterocyclic compounds and their Ni (II), Pd (II), Pt (I), Cu (II) complexes have also been synthesized. Heterocyclic ring system like benzothiazole and few metals like Zn (II,) Pt (II), Cu (II), Ni (II) has also known to posses anti –tumor activity. As nowadays, organo selenium complexes especially of group 12 metals have attracted current attention3. There exit Se—–N intramolecular co-ordination in these complexes which is responsible for its stability. This co-ordination has also shown the possibility that this interaction may play important role in the catalytic cycle of glutathione peroxidase, a selenium containing anti-oxidation enzyme4. Selenium is also known to be anti-mutagenic and anti-carcinogenic agent5. So, attempts have been made to synthesize benzothiazole selenato metallic complexes and screen them for anti-tumor activity using Ehlirch asicites carcinoma (EAC) cells induced in albino mice.
Material and method
All the reaction were carried out under nitrogen atmosphere obtained from nitrogen cylinder using pyragallol solution (for the removal of moisture and oxygen), KOH pellets and concentrated H2SO4 (for the removal of water)6, 7. This nitrogen free of moisture and oxygen was then filled into a balloon fitted with a take off. Solvents were purified by standard procedure and were freshly distilled prior to use. Melting points were recorded by capillary tube method8, 9. IR spectra were recorded on a shimadzu-IR Spectrophotometer using KBr pellets. NMR spectra were carried on Brunker 200 spectrospin using TMS as internal standard. Mass spectra were done using Electron impact (EI) method10, 11, 12. Atomic Absorption Spectroscopy was carried out at Indian bureau of mines, Bangalore, letter no: K-23011/4/chem/2001-2002/analy/bang/OD. The structures of all the compounds were consistent with their analytical and spectral data .Anti-tumor screening was carried out using Ehlirch asicites carcinoma (EAC) Cells induced albino mice brought from Kasturba Medical College, Manipal and were maintained in vivo in mice by i.p every 10 day in our college animal house. The permission of conducting this experiment was obtained from Institutional Animal Ethics Committee, Letter No. KCP/ IAEC – 02/2003.
Synthesis of M[Se(BTA)]2 (IV) Where M = Zn(IV)a, Ni(IV)b,Cu(IV)c, Cd(IV)d, Hg(IV)e.
To a solution of benzothiazole (1.1ml,10mmol) in dry tetrahydrofuran 20 ml was added 1.6 M solution of n-butyl lithium in hexane(6.8ml,11mmol) under nitrogen atmosphere at -78° C, after 1hr of stirring , a orange colour lithated product was obtained13. At this temperature selenium powder (0.8g, 10mmol) was added and stirred for 1hr. Then, the temperature was lowered to -30°C anhydrous MCl2 like ZnCl2, NiCl2, HgCl2, CdCl2, and CuCl2, were added. Stirring was continued for 1hr at this temperature for 18 hrs at room temperature. The resulting solution was filtered and solvent evaporated. The crude solid was recrystallized using organic solvent 1, 4 dioxane to obtain the crystallized complexes M[Se(BTA)]2 . The reaction scheme is shown in fig No. 1
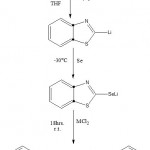 |
Figure 1: Reaction Scheme.
|
IVa: IR (cm-1): Ar. (C – H) st. = 3055.66, (C=N) st. = 1452.14, (C – N) st. = 1312.32, (C – S) st. 1613.16.
1H NMR: 7.34-7.37 (-CH, t, aromatic), 7.44-7.47 (-CH, t, aromatic), 7.78-7.80 (-CH, d, aromatic), 7.94-7.96 (-CH, d, aromatic).
IVb: IR (cm-1): Ar. (C – H) st. = 3062.41, (C=N) st. = 1461.78, (C – N) st. = 1310.39, (C – S) st. 1613.16.
Mass
IVc: IR (cm-1): Ar. (C – H) st. = 3059.51, (C=N) st. = 1455.99, (C – N) st. = 1318.11, (C – S) st. 1621.84.
1H NMR: 7.34-7.39 (-CH, t, aromatic), 7.43-7.49 (-CH, t, aromatic), 7.77-7.81 (-CH, d, aromatic), 7.93-7.98 (-CH, d, aromatic).
IVd: IR (cm-1): Ar. (C – H) st. = 3057.58, (C=N) st. = 1451.14, (C – N) st. = 1309.43, (C – S) st. 1634.38.
1H NMR: 7.33-7.39 (-CH, t, aromatic), 7.43-7.49 (-CH, t, aromatic), 7.76-7.82 (-CH, d, aromatic), 7.93-7.98 (-CH, d, aromatic).
IVe: IR (cm-1): Ar. (C – H) st. = 3057.58, (C=N) st. = 1456.96, (C – N) st. = 1311.36, (C – S) st. 1632.45.
1H NMR: 7.33-7.38 (-CH, t, aromatic), 7.43-7.48 (-CH, t, aromatic), 7.75-7.81 (-CH, d, aromatic), 7.93-7.99 (-CH, d, aromatic).
Table 1: Effect of derivatives on Survival Time on EAC bearing Mice.
| Group number | Design of Experiment | Mean Survival Time (MST) in days | % Increase in Life Span (% ILS) |
| I | Normal Saline 5ml/kg | —– | —– |
| II | EAC only (106 cells/mouse) | 14 | —– |
| III | EAC + vehicle | 14 | —– |
| IV | Zn-Se | 29 | 98.62 |
| V | Ni-Se | 25 | 72.23 |
| VI | Cu-Se | 30 | 36.98 |
| VII | Cd-Se | 15 | 27.39 |
| VIII | Hg-Se | 17 | 16.43 |
Anti –tumor activity
Animals 8-10 week old, albino mice of either sex with an average body weight of 20-25gm were maintained in identical condition and fed with standard food pellets and water. EAC cells were maintained in vivo in the mice by injecting every 10 days.EAC cells of 10 days old mice were used for the studies. Challenge dose of EAC cells was given i.p. at the rate of 1×106 cells/20gm bodyweight of the animal.14 The animals were divided into 13 groups each containing 8 mice .animals of group I-III were kept as saline control (5ml/kgbodyweight i.p), Ehrlich asities carcinoma (EAC) control (1×106 cells/20gm bodyweight per mice i.p), Ehrlich asities carcinoma (EAC) control (1×106 cells/20gm bodyweight per mice i.p) + DMSO (vehicle control) respectively. 5 derivatives were dissolved in DMSO and were administered i.p at the dose of 5microgm / 20gm of body weight in the group (IV-XIII). All the compounds were admistered for 9 days starting 24 hrs after tumor implantation. Four animals from each group were scarified 24 hrs after the last dose and asities fluid volume, packed volume, hematological parameters were noted.15 Mean survival time for remaining four mice of each group was noted for 6 weeks.Asities volumes was noted by taking it in a graduated centrifuge tube and packed cell volume determined by centrifuge at 1000 r.p.m for 5 mins. Viability of asities cells was checked by trypan blue (0.4٪ in normal saline) dye exclusive test and the count were taken in neubauer chamber. The effect of derivatives on tumor growth was monitored by recording the mortality daily for 6 weeks and percentage increase in life span (٪ILS) was calculated.16 An enhancement by 25٪ or more was considered as effective anti-tumor response. Hematological studies were carried out by obtaining blood from tail vein 24 hrs after the last dose. For total blood count blood was drawn into RBC or WBC pipettes dilution in neubauer counting chamber. Hemoglobin concentration was determined by sahli’s hemoglobinometer method.17 Different count of leukocytes DLS was done on freshly drawn blood film using leishmans stain. The most essential parameters that confirm the potency of anti tumor agent are percentage increase in life span (Table no 1), decrease in asicites fluid volume, (Table no 2), increase in RBC, decrease in WBC, increase in lymphocytes18 (Table no 3).
Table 2:Effect of derivatives on Ascites fluid volumr and tumor cell count in a EAC induced Mice
| Group | Design of Experiment | Total Ascites | Packed | % viable |
| Number | fluid volume | tumor cell | cell in | |
| (ml) | volume (ml) | Ascites | ||
| I | Normal Saline 5ml/kg | |||
| II | EAC only (106 cells/mouse) | 7.2 | 3 | 95.72 |
| III | EAC + vehicle | 6.8 | 2 | 92.58 |
| IV | Zn-Se | 5 | 1.2 | 36.23 |
| V | Ni-Se | 4 | 1.53 | 21.72 |
| VI | Cu-Se | 3 | 2 | 43.11 |
| VII | Cd-Se | 4 | 1.32 | 53.42 |
| VIII | Hg-Se | 5 | 3 | 52.88 |
Table 3: Effect of benzothiazole derivates on Haematological parameters in tumor bearing mice.
Group No. |
Design of Experiment | Hb (gm/dl) | RBC Count (X 106) | WBC Count (X 106) | Lymphocyte (%) | Neutrophile (%) | Monocyte (%) |
| I | Normal Saline 5ml/kg | 13 | 5.26 | 6.86 | 58 | 30 | 2 |
| II | EAC only (106 cells/mouse) | 11 | 7.33 | 23.23 | 42 | 74 | 1 |
| III | EAC + vehicle | 10 | 6.58 | 11.22 | 42.9 | 60 | 1 |
| IV | Zn-Se | 10.9 | 6.98 | 9.20 | 75 | 52 | 1.2 |
| V | Ni-Se | 11 | 4.81 | 19.5 | 62.1 | 45 | 1.7 |
| VI | Cu-Se | 11.05 | 5.41 | 14.31 | 48.8 | 22 | 2 |
| VII | Cd-Se | 11.25 | 3.23 | 20.24 | 56 | 24 | 2.5 |
| VIII | Hg-Se | 10.8 | 2.92 | 20.24 | 28.8 | 36 | 1 |
Table 4: Data of various Derrivatives.
| S. No. | Comp Code | Molecular Formula | Mol Wt | M. P. (°C) | % yield | Rf Value |
| 01
02 03 04 05 |
IVa
IVb IVc IVd IVe |
Zn[Se(BTA)2
Ni[Se(BTA)]2 Cu[Se(BTA)]2 Cd[Se(BTA)]2 Hg[Se(BTA)]2 |
491.68
485.00 489.85 538.70 626.90 |
300
190 120 170 120 |
20
30 20 10 10 |
0.34
0.54 0.60 0.32 0.61 |
Result and Discussion
Different selenium complexes were synthesized using benzothiazole (I) which was lithated at 2-position by n-butyl lithium at -78°c under nitrogen atmosphere to give lithated benzothiazole (II). Then chalcogen Se was introduced to this lithated benzothiazole to get lithium benzothiazole selenato ligand BTA Se+ Li– (III). To this was added MCl2 like, ZnCl2, NiCl2, HgCl2, CdCl2, and CuCl2, to yield the final products named benzothiazole selenato metallic complexes (IV) as (IV)A, (IV)B, (IV)C, (IV)D, (IV)E derivatives . The compounds were characterized by, melting point, Rf, (table no5) I.R, NMR, Mass and Atomic Absorption Spectroscopy. Ehlirch asicites carcinoma (EAC) Cells induced albino mice were used to screen them for anti –tumor. All the compounds were admistered daily for 9 days starting 24 hrs after tumor implantation. Four animals from each group were sacrificed 24 hrs after the last dose and various parameters were noted. The assessment of the drug effect was done by couting total packed cell volume (TPCV) and survival time. The compounds (IV)a Zn[Se(BTA)]2 was found to be potent while (IV)b, (IV)c Ni[Se(BTA)]2 ,Cu[Se(BTA)]2, was moderate and (IV)d, (IV)e Cd[Se(BTA)]2 , Hg[Se(BTA)]2 were inactive.
Acknowledgments
I am thankful to my principal, Krupanidi College of Pharmacy for providing every laboratory facilities and also thankful to my teacher’s of the college. I am thankful to Indian Institute of Sciences for providing me guidance especially to Dr. G. Mugesh, Dept of inorganic chemistry.
References
- Khan T. A. Sahajahan, Zaidi S. A.A., Indian Journal of Chemistry, Vol. 36A, 153 (19970.
- Mishra L., Jha A., HidejiIto Kawa, Koichi Takeya, Indian Journal of Chemistry, Vol. 37A, 747-752 (1998).
- Mugesh G., Singh H. B., Patel R. P. And Butcher R. J., Inorganic Chemistry, Vol. 37, no. 11, 2663-2668 (1998).
- Twaoka M., and Tomoda S., J. Am. Chem. Soc., Vol. 118, 8077-8084 (1996).
- NIH Clinical Center, facts about Dietary Supplements, http:// www.cc.nih.gov/ccc/supplements/selen.html.
- Brain, Furniss, Anthony J., Hanna fond, Vogel’s Textbook of Practical Organic Chemistry, ELBS London, 236-238 (1959).
- Furniss, Hannafords, Rogger’s, Smith, Tatchell, Volgel’s Textbook of practical organic Chemistry, 4th ed., ELBS London, 268-274.
- Silverstein R. M., Clayton B. G., Morril T. C., Spectroscopic Identification of Organic Compounds, 5th ed., John Willy, New York, 100-130 (1991).
- John R. Dyer, Application of Absorption Spectroscopy of organic compounds, 10th ed., Prentice-Hall of India, New Delhi, 32-38 (1997).
- William Kemp, Organic Spectroscopy, 5th ed., Macmillan publishing Co., New York, 56-86 (1991).
- Silverstein R. M., Bassler C. G., Morril T. C., Ultra Violet Spectroscopic Identification of Organic Compounds, 5th ed., John Willy, New York, 289-300 (1991).
- Remington; the Science and practice of Pharmacy, 19th ed., 578-579 (1995).
- Chikashita H., Megumi, Ishibaba, Keiji and Itoh K., Bull. Chem. Soc. Jpn., vol. 61, No. 10, 3637-3648 (1998).
- Bradshaw t. D., Schultz R. J., Paull K. D., Kelland L., Wilson A., Garner C., Wrigley S., Steven M. F. G., ………………………………………….
- Khanam J. A., Bag S. P., Sur B., Sur p., Indian Journal of Pharmacology, Vol. 29 157-161 (1997).
- Srikant K., Debanth B., Nayaki S. S., Jha T., Indian journal of Chem., vol. 35, 172-177 (2002).
- Mazumder U. K., Gupta N., Sur M., Bhattyacharya S., Indian Journal of Pharmaceutical Science, Vol. 5, 386-390 (2001).
- Mazumder U. K., Gupta N., Maiti S., Mukherjee D., Indian Journal of Experimental Biology, Vol.35, 473-477 (1997).

This work is licensed under a Creative Commons Attribution 4.0 International License.





