Manuscript accepted on : May 21, 2008
Published online on: 06-02-2016
Khalid M. Al-Ghamdi, Jazem A. Mahyoub And Mostafa S. Saleh*
1Department of Biological Sciences, Faculty of Science, King Abdulaziz University. 2Mosquito Research Lab., Jeddah Saudi Arabia.
ABSTRACT: Susceptibility levels of Culex pipiens L. mosquito larvae to the conventional insecticides Actikil and Pesguard as well as non-conventional ones the insect growth regulator Dudim, the bioinsecticide Bacilod and the plant extract Neem oil were determined. Taking LC50 values (concentration which to kill 50% of larvae) into consideration, mosquito larvae of C. pipiens were more susceptible to the pyrethroid Pesguard (0.048 ppm) than the organophosphate Actikil (0.052 ppm) and the biocide Bacilod (0.13 ppm) by about 1.1 and 2.7 folds, respectively. According to IC50 values (concentration which to inhibit the emergence of 50% of adults), the IGR Dudim (0.0003 ppm) proved to be more effective against mosquito larvae of C. pipiens than the plant extract Neem oil (70 ppm). Variation in the susceptibility status of the present mosquito larvae may be attributed to the differential mode of action of the compounds tested and its effective concentrations. On the other hand, different levels of potentiation and additive effects were obtained when the present compounds were applied jointly against mosquito larvae of C. pipiens.
KEYWORDS: Mosquito larvae; Susceptibility status; larval bioassay; joint action
Download this article as:| Copy the following to cite this article: Al-Ghamdi K. M, Mahyoub J. A, Saleh M. S. Evaluation of some conventional and non-conventional insecticides against mosquito larvae of Culex pipiens L. Biosci Biotechnol Res Asia 2008;5(1) |
| Copy the following to cite this URL: Al-Ghamdi K. M, Mahyoub J. A, Saleh M. S. Evaluation of some conventional and non-conventional insecticides against mosquito larvae of Culex pipiens L. Biosci Biotechnol Res Asia 2008;5(1). Available from: https://www.biotech-asia.org/?p=6522 |
Introduction
Drawbacks associated with widespread use of conventional insecticides to control mosquitoes have not only resulted in the development of resistance in many species of mosquito vectors, but have also caused environmental pollution. Therefore, more attention has been recently paid to the use of non-conventional insecticides such as insect growth regulators IGRs, bioinsecticides and plant extracts for mosquito control in different parts of the world (Saleh and Wright, 1989; Canyon and Hii, 1999; Cornel et al., 2000; Paeporn et al., 2005; Chowdhury et al., 2007).
The present study was planned in part to evaluate the biological activity of some conventional and non-conventional insecticides against C. pipiens L., the dominant mosquito species in Jeddah governorate, Saudi Arabia. Additional tests were also conducted to study the possible larvicidal effectiveness of the tested insecticides when applied jointly against mosquito larvae of C. pipiens.
Materials and Methods
Mosquito strain
Tests were performed on a field strain of C. pipiens raised from wild larvae, collected from Jeddah, Saudi Arabia, and had been maintained in the laboratory under controlled conditions of 27 ± 1ºC and 70 ± 5% R.H., with a 14:10 (L:D) photoperiod.
Compounds tested
The following compounds were used:
1-The organophosphate insecticide Actikil (5% EC); O-(2-Diethylamino-6-methyl-4-pyrimidinyl)-O,O-dimethyl phosphorothioate.
2-The pyrethroid insecticides Pesguard Fg 161 (% EC);
1,3,4,5,6,7- hexahydro-1,3dioxo-2H-isoindol-2-yl) methyl 2,2- dimethyl-3-(2-methyl-1-propenyl) cyclopropanecarboxylate and alph-Cyano-3-phenoxybenzyl 2,2- dimethyl-3-(2-methylprop-1-enyl) cyclopropanecarboxylate.
3-The bacterial insecticides Bacilod, a wattable powder formulation of Bacillus thuringiensis var. israelensis (B.t.i.), kindly supplied by Dr. Kh. Al-Ghamdi, Fac. of Science, King Abdulaziz Univ. The material had a potency of 5000 IU/mg.
4-The insect growth regulator Dudim (4 G%); 1-(4-chlorophenl)-3-(2,6-difluorobenzoyl)-urea.
5-The plant extract Neem oil (Azadirachta indica), kindly supplied by Dr. M. A. Khan, Dept. of Zoology, Saifia Science college Bhopal, India. The stock solution of the plant extract was prepared by adding 1ml of it to 99 ml of distilled water containing 0.5% triton X-100 as an emulsifier to ensure complete solubility of the extract in water. Series of concentrations were prepared in distilled water.
Larval bioassay
The standard WHO larval susceptibility test method (WHO, 1981) was used. Treatments were carried out by exposing early 4th instar larvae of C. pipiens to various concentrations of the tested compounds for 24 hr, in groups of glass beakers containing 100 ml of tap water. Five replicates of 20 larvae each per concentration, and so for control trials were set up. The larvae were given the usual larval food during these experiments. Larval mortalities were recorded at 24 hr post-treatment for the chemical insecticides Actikil and Pesguard as well as the biocide Bacilod. In the case of the IGR Dudim and the plant extract Neem oil, cumulative mortalities of larvae and pupae were recorded daily. Live pupae were transferred to untreated water in new beakers for further observation, i.e. normal emergence, presence of morphologic abnormalities or death. Partially emerged adults or these found completely emerged but unable to leave the water surface were recorded and scored as dead. Therefore, the biological effect of Dudim and Neem oil was expressed as the percentage of larvae that do not develop into successfully emerging adults, or the inhibition of adult emergence (WHO, 2005). Log concentration-probability regression lines were drawn for the tested compounds and statistical parameters were also calculated using the method of Litchfield and Wilcoxon (1949).
Joint action testes
Values of IC20, IC30 and IC40 (concentrations which to inhibit the emergence of 20, 30 and 40% of adults, respectively) were obtained from the toxicity line of the IGR Dudim. The concentrations corresponding to these values were prepared. The combinations were applied at the above sublethal concentrations of Dudim with the IC40 value of the plant extract Neem oil. Another trials were conducted using the bacterial insecticide Bacilod at the LC40 value (concentration which to kill 40% of larvae) with the chemical insecticides Actikil and Pesguard at LC20, LC30 and LC40 levels. Five replicates of 20 larvae were conducted for each mixture. The joint action of different mixtures was expressed as the coeffective factor (C. F.) according to the equation given by Mansour et al. (1966) as follows:
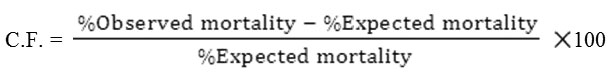
This factor was used to differentiate results into three categories. A positive factor of 20 or more is considered potentiation; a negative factor of 20 or more means antagonism and intermediate values between -20 and +20 indicate only additive effects.
Results and Discussion
Susceptibility levels of C. pipiens mosquito larvae following treatment with different concentrations of the chemical insecticides Actikil and Pesguard as well as the bacterial insecticide Bacilod are shown in Table 1. The effective concentrations of the above compounds against 4th larval instars ranged from 0.02 – 0.15 ppm, 0.02 – 0.2 ppm and 0.05 – 0.5 ppm, respectively. The corresponding larval mortalities for these compounds were 12 – 93%, 23 – 92% and 15 – 92%. Taking LC50 values (concentration which to kill 50% of larvae) into consideration, the records showed that the pyrethroid Pesguard (0.048 ppm) proved to be the most effective compound, followed by the organophophate Actikil (0.052 ppm) while the biocide Bacilod (0.13 ppm) was the least effective. In other words, the results indicate that mosquito larvae of C. pipiens were more susceptible to Pesguard than Actikil and Bacilod by about 1.1 and 2.7 folds, respectively. However, it can be concluded that the change in the susceptibility levels of the present mosquito larvae is possibly due to the differential mode of action of the test compounds and its effective concentrations. The fluctuations in the percentage mortalities obtained for the different concentrations of different compounds tested against the present mosquito larvae support this conclusion (Saleh and Aly, 1987; Canyon and Hii, 1999; Nazni et al., 2005).
Table 2 shows the percentage of mortalities of larvae and pupae as well as the inhibition of adult emergence, following treatment with different concentrations of the IGR Dudim and the plant extract Neem oil. In general, 2 – 30% and 11 – 65% larval mortalities were obtained when the 4th instar larvae of C. pipiens were treated with the above compounds, respectively. The biological effects were often manifested by the formation of a type of larval-pupal intermediate (Fig. 1-A). Most pupae retained the 4th instar cuticle, but those that pupated successfully often died either before the adult emerged or as “albino” pupae (Fig. 1-B), that is, they lacked the hardening and darkening of the cuticle (Bridges et al., 1977). Many adults emerged incompletely or left their tarsi attached in the pupal exuvia (Fig. 2) (Saleh et al., 1981; Al-sharook et al., 1991). Therefore, in the present work, cumulative mortality during larval development to pupae and adults have been taken as a criterion for the evaluation of the tested compounds as they have more juvenilizing effects than toxic mode of action (WHO, 2005). However, the effective concentrations of Dudim and Neem oil ranged from 0.0001 – 0.005 ppm and 40 – 150 ppm, respectively. The corresponding percentages of inhibition of adult emergence were in respect 22.5 – 94.6% and 23.4 – 92.5%. Taking IC50 values (concentrations which to inhibit the emergence of 50% of adults) into consideration, Dudim (0.0003 ppm) proved to be more effective against C. pipiens than Neem oil (70 ppm). Laboratory and field studies in this respect were carried out by several authors to determine the susceptibility level of different mosquito species to insecticides (Baruah and Dusl, 1996; Sagar et al., 1999; Cornel et al., 2000; Chowdhury et al., 2007). However, it can be concluded that the tests on susceptibility status of mosquitoes to insecticides in any area will provide baseline data for planning control programmes and making decisions about insecticide usage in these areas (Paeporn et al,., 2005).
Table 3 shows the percentage of expected and observed inhibition of adult emergence, coeffective factor (C. F.) and the type of interaction resulted from the combinations of the plant extract Neem oil with the IGR Dudim against the mosquito C. pipiens. The combinations were applied at the IC40 value of Neem oil (58 ppm) and IC20, IC30 and IC40 levels of Dudim (0.0001, 0.0002 and 0.0003 ppm, respectively). Taking the values of coeffective factor (C.F.) into considerations, the results showed that all combinations of Neem oil and Dudim produced different levels of additive effects (C. F. = 5, -11.4 and -11.2) against mosquito larvae and reflected by the inhibition of adult emergence. Variation in the levels of additive effects among the test mixtures may be attributed to the differential mode of action of the present compounds and the concentrations tested (Saleh et al., 2003).
Table 4 shows the effect of combination of the bacterial insecticide Bacilod with the chemical insecticides Actikil and Pesguard against mosquito larvae of C. pipiens. The combinations were applied at the LC40 levels of Bacilod (0.1 ppm) and LC20, LC30 and LC40 levels of Actikil (0.028, 0.035 and 0.044 ppm) and Pesguard (0.019, 0.027 and 0.036 ppm). In general, values of C.F. indicated that Bacilod in combination with the OP insecticide Actikil (C.F. = 33.3; 21.4 and 22.5) and the pyrethroid Pesguard (C.F. =16.7, 19.0 and 13.8) produced different levels of potentiation and additive effects according to the levels of sublethal concentrations combined. Similar findings have been reported by Saleh et al. (1990) who found that the joint action of the pathogen B.t.i. with the chemical insecticides malathion, dursban and fenvalerate against mosquito larvae of A. aegypti resulted in potentiation and additive effects. However, it has been suggested that the potentiation of toxicity may be due to the larvae survived partially-lethal treatments of the pathogen B.t.i. were often weakened sufficiently to become more susceptible to the toxic effects of the tested chemical insecticides (Kelada and Shaker, 1988). Generally, Long term follow-up studies are needed to determine how the environmental conditions affect the effectiveness of such compounds when applied jointly for field control measures.
Table 1: Susceptibility levels of mosquito larvae of C.pipiens to Actikil, Pesguard and Bacilod following continuous exposure for 24 hr.
| compound | Effective
Concentrations (ppm) |
Larval mortality a
(% ) |
Statistical calculations b | |||
| Slope
function |
LC50
(ppm) |
fLC50 | slope | |||
| Actikil | 0.02-0.15 | 12-93 | 2.19 | 0.052 | 1.16 | 2.94 |
| Pesguard | 0.02-0.2 | 23-92 | 2.9 | 0.048 | 1.24 | 2.16 |
| Bacilod | 0.05-0.5 | 15-92 | 2.5 | 0.13 | 1.2 | 2.52 |
a Five replicates, 20 larvae each; control mortalities ranged from 0.0-3%.
b Litchfield and Wilcoxon (1949).
Table2. The biological effects of the IGR Dudim and the plant extract Neem oil on the developmental stages of C. pipiens.
| compound | Concentrations
(ppm) |
Larval mortality a
(% ) |
Pupae
produced (%) |
Adult emergence | LC50
(ppm) |
||
| Total | Inhibition | ||||||
| observed | Corrected b | ||||||
|
Dudim |
0.0001 | 2 | 98 | 72 | 28 | 22.5 |
0.0003 |
| 0.0004 | 10 | 90 | 40 | 60 | 56.5 | ||
| 0.007 | 9 | 91 | 27 | 73 | 70.9 | ||
| 0.001 | 3 | 97 | 18 | 82 | 80.7 | ||
| 0.005 | 30 | 70 | 5 | 95 | 94.6 | ||
| control | 4 | 96 | 93 | 7 | |||
|
Neem oil |
40 | 11 | 89 | 72 | 28 | 23.4 |
70 |
| 60 | 15 | 85 | 56 | 44 | 40.4 | ||
| 80 | 30 | 70 | 43 | 57 | 54.7 | ||
| 100 | 41 | 59 | 20 | 80 | 78.7 | ||
| 150 | 65 | 35 | 7 | 93 | 92.5 | ||
| control | 3 | 97 | 94 | 6 | |||
a Five replicates, 20 larvae each.
b Corrected with Abbott’s formula (Abbott, 1925).
Table 3: The joint action of the IGR Dudim and the plant extract Neem oil against 4th instar larvae of C. pipiens.
| Compound mixtures
and IC values |
Concentrations
Used (ppm) |
Inhibition of adult emergence
(%) |
C.F.* |
Type of interaction | |
| Expected | Observed | ||||
| Neem oil+Dudim | |||||
| IC40+IC20 | 58+0.0001 | 60 | 63 | 5 | (++) |
| IC40+IC30 | 58+0.0002 | 70 | 62 | -11.4 | (++) |
| IC40+IC40 | 58+0.0003 | 80 | 71 | -11.2 | (++) |
* Coeffective factor (Mansour et al., 1966).
(++) Additive effect.
Table 4: The joint action of the bacterial insecticide Bacilod with the chemical insecticides Actikil and Pesguard against 4th instar larvae of C. pipiens.
| Compound mixtures
and IC values |
Concentrations
Used (ppm) |
Larval mortality
(%) |
C.F.* | Type of interaction | |
| Expected | Observed | ||||
| Bacilod+Actikil | |||||
| IC40+IC20 | 0.1+0.028 | 60 | 80 | 33.3 | (××) |
| IC40+IC30 | 0.1+0.035 | 70 | 85 | 21.4 | ××) |
| IC40+IC40 | 0.1+0.044 | 80 | 98 | 22.5 | (××) |
| Bacilod+Pesguard | |||||
| IC40+IC20 | 0.1+0.019 | 60 | 70 | 16.7 | (++) |
| IC40+IC30 | 0.1+0.027 | 70 | 83 | 19.0 | (++) |
| IC40+IC40 | 0.1+0.036 | 80 | 91 | 13.8 | (++) |
* Coeffective factor (Mansour et al., 1966)
(××) potentiation
(++) Additive effect
 |
Figure 1: Abnormalities in the developmental stages of C. pipiens after treatment with Neem oil or Dudim. A- Larval – pupal intermediate showing larval siphon (a) and pupal trumpets(b); B- Unmelaninized pupa (albino pupa).
|
 |
Figure 2: Adults of C. pipiens (A, female; B, male﴿ failed to emerge from the pupal skins after larval treatments with Neem oil or Dudim.
|
References
- Al-Sharook, Z.;K. Balan; Y. Jianhg and H. Rembold. Insect growth inhibitors from two tropical Meliaceae effect of crude seed extracts on mosquito larvae. J. Appl. Ent. 111, 425-430 (1991).
- Baruah, I. and S.C. Dusl. Evaluation of Methoprene (Altosid) and Diflubenzuron (Dimilin) for control of mosquito breeding in Tezpur (Assam). Indian J. Malariol. Jun, 33(2): 61-66 (1996).
- Bridges, A.C.; J. Coke; J. K. Olson and R. T. Mayer. Effects of new fluorescent insect growth regulators on larval instars of Aedes aegypti. Mosquito News.37: 227-233 (1977).
- Canyon D. and Hii J. Insecticide susceptibility status of Aedes aegypti (Diptera: culicidae) from Towncvile. Aust. J. Ent. 38(1): 40-43 (1999).
- Chowdhury N.; Bhattachajee I.; Laskar S. and Chandra. G. Efficacy of Solanum villosum Mill. (Solanaceae: Solanales) as a biocontrol agent against fourth instar larvae of Culex quinquefasciatus Say. Turk J. Zool. 31:365-370 (2007).
- Cornel, A. J.; M.A. Stanich; D. Farley; F. S. Mulligan and G. Byde. Methoprene tolerance in Aedes nigromaculis in Fresno country, California. J. Am. Mosq. Control Assoc., 16(3):223-228 (2000).
- Kelada, N.L and N. Shaker. Toxicity of three chemical insecticides in combination with Bacillus spp. against mosquito larvae. Insect. Sci. Applic. 9:583-588 (1988).
- Litchfield, J.T. and E. Wilcoxon. A simplified method of evaluating dose-effect experiments. J. Phar. Exp. Ther.96, 99-113 (1949).
- Mansour, N. A.; M. E. Eldafrawi ; A. Toppozada and M. Zeid. Toxicological studies on the Egyption cotton leaf worm Prodenia litura. VI. Potentiation and antagonism of carbamate insecticides. J. Econ. Entomol. 59 : 307- 311 (1966).
- Nazni, W.A.; H.L. Lee and A.H. Azahari. Adult and larval insecticide susceptibility status of Culex quinquefasciatus (Say) mosquitoes in Kuala Lumpur Malaysia. Tropical Biomedicine 22(1): 63-68 (2005).
- Paeporn, P.; S. Kasin; S. Sathantriphop and S. Sangkitporn. Insecticides Susceptibility of Aedes aegypti in Tsunami-affected Areas in Thailand. Dengue Bull. 29: 210-213 (2005).
- Sagar, S.K,; S.S. Sehgal and S.P. Agarwala. Bioactivity of ethanol extract of karanja Progamia glabravent seed coat against mosquitoes. J. Commun Dis. 31(2):107-111 (1999).
- Saleh, M.S.; I.A. Gaaboub and Sh. M.I. Kassem. Larvicidal effectiveness of three controlled release formulations of Dursban and Dimilin on Culex pipiens and Aedes aegypti. J. Agric. Sci. Camb. 97:87-96 (1981).
- Saleh, M.S. and M.I. Aly. The biological effects of three insect growth regulators on Culex pipiens L. Anz. Schadlingskde., pflanzenschutz , Umweltschutz , 60 : 34-37 (1987).
- Saleh , M.S. and R.E. Wright. Effects of the IGR cyromazine and the pathogen Bacillus thuringiensis var. israelensis on the mosquito Aedes epacticus. J. Apple Entomol ., 108 : 381 – 385 (1989).
- Saleh M.S. , N.L Kelada and M. Abdeen . Factors affecting efficacy of Bacillus thuringiensis H-14 against mosquito larvae with special reference to the joint action of the pathogen with three chemical insecticides against mosquitoes. Anz . F. Schadlingsk pflanzenschutz , Umweltschutz , 63 : 10-13 (1990).
- Saleh, M.S.; N.L.Kelada; Fatma A. El-Meniawi and H.M. Zahran. Bacillus thuringiensis var. israelensis as sustained-release formulations against the mosquito Culex pipiens with special reference to the larvicidal effects of the bacterial agent in combination with three chemical insecticides. Alex. J. Agric. Res. 48(1):53-60 (2003).
- World Health Organization.Instructions for determining the susceptibility or resistance of mosquito larvae to insecticides. WHO/VBC. 81. 807: 1-6 (1981).
- World Health Organization. Guidelines for laboratory and field testing of mosquito larvicides. WHO/ CDS/ WHOPES/ CDPP/13 (2005).

This work is licensed under a Creative Commons Attribution 4.0 International License.





