Manuscript accepted on :
Published online on: 07-01-2016
Plagiarism Check: Yes
M. A. Deepa1, T. Suresh1, T. Dhanabal2, P. S. Mohan2 and V. Narmatha Bai1*
1Tissue Culture Laboratory, Department of Botany, Bharathiar University, Coimbatore - 641 046 (India) 2Department of Chemistry, Bharathiar University , Coimbatore - 641 046 (India)
ABSTRACT: A New compound possessing benzoid skeleton has been isolated from the leaves of Salacia beddomei a potential medicinal plant. Structure of the isolated compound has been proposed on the basis of spectroscopic analysis. The antimicrobial, antifeedant and isecticidal properties of the isolated terpenoid were also discussed.
KEYWORDS: Salacia beddomei; antimicrobial activity; antifeedant activity; tepenoid
Download this article as:| Copy the following to cite this article: Deepa M. A, Suresh T, Dhanabal T, Mohan P. S, Bai V. N. A New Bezoid System With Antifeedant And Antimicrobial Properties From The Leaves Of Salacia Beddomeri Gamble. Biosci Biotechnol Res Asia 2003;1(2) |
| Copy the following to cite this URL: Deepa M. A, Suresh T, Dhanabal T, Mohan P. S, Bai V. N. A New Bezoid System With Antifeedant And Antimicrobial Properties From The Leaves Of Salacia Beddomeri Gamble. Biosci Biotechnol Res Asia 2003;1(2). Available from: https://www.biotech-asia.org/?p=3405 |
Introduction
Salacia beddomei Gamble1 (Korandi) is a woody liana endemic to Western Ghats. The plant as a whole and especially the root is an important source of antidiabetic drug that finds application in traditional and tribal medicines in India. The plant is found rich in terpenoids. Salacianone, salacinol, betulin, pristimerin and β- sitosterol were isolated from the stem break of Salacia beddomei 2. The terpenoids isolated from the plants were known to pharmaceutically important bioactive compounds. Two lupane triterpenoids 20,29-epoxysalacianone and 6β-hydroxysalacianone together with betulin and friedelanone were reported from the stem bark of S. beddomei3-4. Salacinol is a most potent α -glucosidase inhibitor isolated from Salacia5. In the present study an aromatic based compound has been isolated from the leaves of S. beddomei and structure of the compound has been proposed on the basis of spectral studies.
Experimental
Extraction and isolation
The leaves of Salacia beddomei Gamble were collected from a five-year-old plant grown in the Medicinal plant conservation park of “Center for Indian Medical Heritage” (CIMH), Kanjicode, Kerala. The air-dried leaf powder was extracted with ethyl acetate and subjected to thin layer chromatographic analysis. The crude extracts were subjected to column chromatography and the compound was separated by eluting in petroleum ether : ethyl acetate. Melting points were determined by Boetieus microheating table and Melter FP5 apparatus and are uncorrected. They are expressed in degree centigrade (°C). The IR spectra were recorded on Shimadzu-8201 (PC) FT IR instrument using KBr disc and the adsorption frequencies are expressed in reciprocal centimeters. The ¹H-NMR was recorded on Varian AMX-400 MHz spectro-photometer, using tetramethylsilane (TMS) as an internal reference. Mass spectra were recorded on Jeol JMS-D 300 (70ev) mass spectrometer.
Result and Discussion
The thin layer chromatographic analysis of the ethyl acetate extracts of the leaves has shown that two spots with traces of impurities. The crude extracts were subjected to column chromatography and the major compound was separated by eluting in petroleum ether : ethyl acetate fractions. 0.3129 gms of compound was obtained as an amorphous white powder in 100% ethyl acetate elution. The compound gave positive result for libermann-burchard reaction characteristic for terpenoids. The compounds melted at 162°C-165° C; IR (KBr) [γmax cm-1] : 3830, 3747, 2923, 2856, 1693, 1647 and 1045; ¹H-NMR (CDCl 3) [δ ppm]; δ 0.9[d, 3h, CH 3 J= 8.04 Hz], δ 1.6 [s, 3H,CH 3], δ3.55 [s,3H,O Me], δ3.75 [ q, 1H, CH, J=5.02 Hz], δ4.0 [s, 1H, CH], δ4.85[s,2H,- CH2-], δ 5.6[bs, 1H, -OH], δ 7.1 [s, 1H, Ar-OH], δ 7.5 [s, 1H, Ar-H]; MS(m/z): 248, 187, 173, 149, 97, 69, 44(100)
With the mass spectrum of the compound showing the molecular ion peak at m/z 248 and other spectroscopic data in expected to posses the molecular formula C14H16 O4. With the help of the above spectral data, the compound having 6-hydroxy-8-hydroxymethyl-7- methoxy -3,4- dimethyl-4H-naphthalen-1-one skeleton was proposed for the isolated compound, which was similar to the basic skeleton of an aromatic terpene reported from Tripterygium wilfordii6.
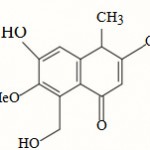 |
Scheme 1 |
6-Hydroxy-8-hydroxymethyl-7-methoxy-3,4-dimethyl-4H-naphthalen-1-one
Numerous terpenoids isolated from Celastraceae members possess a similar structure in their basic skeleton as the one reported in the present study.
Antimicrobial activity
Antibacterial activity – The antibacterial activity of the isolated compound has been evaluated. The compound was screened for activity against Bacillus subtilis, Escherichia coli, Klebsiella pneumonia, Pseudomonas phosphorescence, and Aeromonas hydrophila at 0.1%, 0.5% and 1% concentration by agar well diffusion method8. Results were compared with the standard drugs (streptomycin and ciprofloxacin). The compound showed appreciable zone of activity at 0.5(9.2, 4.1, 11.5, 5.1 and 9.1 mm) and maximum zone of activity at 1% (10.4, 13.2, 14.5, 6.7 and 10.8 mm) concentrations against the test pathogens respectively.
Antifungal activity- The antifungal activity of the compound isolated has been evaluated using agar well diffusion method. The compound has been tested against Aspergillus niger, Alternaria alternata and Fusarium oxysporum at 0.1, 0.5% and 1% concentrations . The compound exhibited significant activity against all the three tested fungi. The compound showed a maximum zone of inhibition 14.7, 4 and 17.1 at 0.5% concentrations and 15.7, 8.1 and 19.2 mm at 1% concentrations against the test pathogens respectively.
Antifeedant and insecticidal activity – The antifeedant activity of the compound against the cotton leaf work Spodoptera litura has been studied using no choice test9. The compound at 0.5% (64%) and 1% (83%) concentration showed maximum antifeedant activity against the IV instar larvae of S. litura and 70% mortality was recorded with 1% extracts.
References
- Gamble, G., Flora of Madras, Shree Sarawathy Press Ltd. Calcutta, (1935)
Hisham, A., Jaya Kumar, G., Fujimoto, Y., and Hara, N., Phytochemsitry 40(4),
1227, (1995) - Hisham, A., Jaya Kumar, G., Fujimoto, Y., and Hara, N., Phytochemsitry 42(3), 789,
(1996) - Hisham, A., Jaya Kumar, G., Fujimoto, Y.,and Hara, N., Phytochemsitry 43 (4), 843,
(1996) - Yoshikawa, M., Murakami, T., Shimada,H., Matsuda, H., Yamahara, J., Tanabe, G., and Muraoka, O., Tetrahedron Lett., 38(48), 8367, (1997)
- Nakano, K., Oose, Y., Matsuda, Y., Kamada, H., and Takaishi, Y. Phytochemistry, 45(2), 293, (1997)
- Aneja, K.R., Microbiology, Plant Pathology and Tissue Culture Wishwa Parkashan, New Delhi, India (1993)
- Belles, X., Campus, F., Coll, J., and Piulachs, 7M.D. J.Chem. Ecol., 11, 1439, (1985)

This work is licensed under a Creative Commons Attribution 4.0 International License.





