Manuscript accepted on :
Published online on: --
Plagiarism Check: Yes
Synthesis And Antimicrobial Activity Of Some New Thiazolidinones Containing N-Methyl Piperazine
H. S. Patel*, H. J. Mistry and H. D. Desai
Department of Chemistry, S. P. University, Vallabh Vidyanagar - 388 120, Gujarat India.
ABSTRACT: 4-(4’-sulphanilyl)-1-methyl piperazine (2) has been prepared by the reaction of N-acetyl sulphanilyl chloride (ASC) with 1-methyl piperazine followed by the hydrolysis of the product with ethanolic HCl. This compound (2) under go condensation with aromatic aldehydes to give the corresponding Schiff’s base (3a-h). The Schiff’s base (3a-h) on cyclocondensation reaction with thioglycolic acid gives 4-thiazolidinones. Biological screening of the prepared compounds has been carried out on some strains of bacteria.
KEYWORDS: Schiffs base; thiazolidinones; antimicrobial activity
Download this article as:| Copy the following to cite this article: Patel H. S, Mistry H. J, Desai H. D. Synthesis And Antimicrobial Activity Of Some New Thiazolidinones Containing N-Methyl Piperazine. Biosci Biotechnol Res Asia 2003;1(1) |
| Copy the following to cite this URL: Patel H. S, Mistry H. J, Desai H. D. Synthesis And Antimicrobial Activity Of Some New Thiazolidinones Containing N-Methyl Piperazine. Biosci Biotechnol Res Asia 2003;1(1). Available from: https://www.biotech-asia.org/?p=3376 |
Introduction
4-thiazolidinone has been extensively investigated by the organic chemists due to their close association with various types of biological activities1-4. Thiazolidinones are of particular interest because of their use as local anesthetics5.
Piperazine derivatives find their wide clinical applications in the therapy of functional diseases and exhibit anthelmintic, antibacterial and insecticidal activities6. Moreover, piperazine derivatives (especially 1-methyl piperazine) are known to possess biological activities such as antifilarial, neuroleptic, antihistaminic, antiviral, CNS depressant, antipsychotic and antiinflammatory7. Incorporation of the thiazolidinone moiety into 1-methyl piperazine is expected to modify their biological activities.
Since the antibacterial effect of Sulphanilamide has been attributed to the presence of a sulphonamide group (-SO2NH2) and an –NH2 group in the para- position. So it was of interest to study the effect of fixation of these groups to the piperazine moiety.
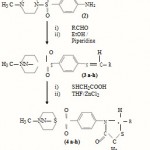
This interest has prompted us to extend this study to include the effect of the introduction of the well-known thiazolidinone nuclei instead of –NH2 group into the piperazine nucleus. Eight substituted 4-thiazolidinones have been prepared and their biological activity has been screened. The research work is scanned in SCHEME-1.
Antibacterial activities
Antibacterial activities of all the compounds were studied against Gram-positive bacteria (Bacillus subtillis and Staphylococcus aureus) and Gram-negative bacteria (E.coli and Salmonella typhi) at a concentration of 50µg/ml by Agar cup plate method. Methanol system was used as control in this method. Under similar condition using penicillin as a standard for comparison carried out control experiment. The area of inhibition of zone is measured in cm. Compounds 4c, 4d and 4h were found more active against the above microbes. Other compounds found to be less or moderate active than Penicillin (Table – 2).
Experimental
Melting points were determined in open capillary tubes and are uncorrected. The IR spectra were recorded in KBr pellets on a Nicolet 400D spectrometer and 1H NMR spectra in CDCl3 on Hitachi R-1500, 60 MHz spectrometer using TMS as an internal standard. The required N-acetyl sulphanilyl chloride was prepared by reported method8. All chemicals used were of laboratory grade.
Preparation of 4-(4’-acetylamino benzene sulphonyl)-1-methyl piperazine (1): General Procedure:
1-methyl piperazine (0.05 mole) was dissolved in a mixture of 40ml anhy. Acetone and 1ml of dry pyridine in 250 ml flask and 11.67 gms (0.05 mole) of pure N-acetyl sulphanilyl chloride (ASC) was slowly added into it. Sodium bicarbonate was added as an acid acceptor. The reaction mixture is set aside overnight and almost pure. 4-(4’- acetylamino benzene sulphonyl) –1- methyl piperazine is filtered off and washed with cold water and air-dried. It was then recrystallized from methlylated sprit to give white product (1) in 65% yield.
Preparation of 4-(4’-sulphanilyl) – 1- methyl piperazine (2) :
General procedure:
4-(4’-acetylaminobenzene sulphonyl)-1-methyl piperazine (1) was hydrolyzed by refluxing with 75 ml of ethanol containing 15ml of concentrated HCl for 4-5 hours. It was then poured into ice-cold water and finally made just alkaline with liq. ammonia. The resultant product 4-(4’ sulphanilyl)-1- methyl piperazine (2) is filtered off and washed with water and air-dried. It was then recrystallized from ethanol to give product (2) in 60% yield.
Preparation of Schiff’s bases (3a-h).
General procedure:
A mixture of equimolar amount (0.01 mole) of hydrolyzed product (2) and the aromatic aldehyde in ethanol (40ml) and piperidine (0.3ml) was refluxed for 3 hours on a water bath. The reaction mixture was concentrated, cooled and it was poured into water and the solid obtained was filtered and recrystallized from ethanol to give the schiff’s base (3a-h). It was obtained in 50-55% yield.
 |
Scheme 1 |
Preparation of 4-thiazolidinones (4a-h)
General procedure
A mixture of schiffs base (0.01 mole) in THF (30ml) and mercaptoacetic acid (0.01 mole) with a pinch of anhydrous ZnCl2 was refluxed for 12 h on a water bath. The solvent was then removed to get a residue, which was dissolved in benzene and passed through a column of silica gel using benzene: chloroform (8:2, v/v) mixture as eluent. The elute was concentrated and the product crystallized form alcohol (50-60%). All the compounds were characterized by analytical and spectral data (Table –1) of the compound is assigned in Scheme –1.
Results and Discussion
N-acetyl benzene sulphonyl chloride (ASC) was condensed with 1-methyl piperazine according to the method described in Bernstein and Rothstein9. The resulting compound (1) was hydrolyzed by ethanolic HCl and yields the corresponding 4-(4’-sulphanilyl) –1-methyl pirerazine. Alcoholic solution of (2) was reacted with aromatic aldehydes in the presence of piperidine as a catalyst to afford Schiff’s base (3a-h). The structures of these compounds (3a-h) have been established by their elemental analysis and spectral data. The IR spectra of these compounds showed absorption bands at 1630 cm-1 (for –CH=N group) and at 1175 cm-1 (for – SO2N group)11.
The cycloaddition reaction of thioglycolic acid on azomethine group (-CH=N group) in (3a- yield the corresponding substituted 4-Thiazolidione (4a-h). Schiff’s bases generally give strong band at 1630-1635cm-1 (-CH =N group). This band is almost vanish in the spectrum of thiazoldin-4-one and a new strong band at 1710-1730 cm-1 was observe which is a characteristics band of the carbonyl stretching frequency for thiazolidinone ring system.
The C,H,N,S analysis of all the compounds of the series are presented in Table – 1. The values are consistent with their predicted structure (Scheme – 1).
Acknowledgement
We wish to thank Dr. R. M. Patel, Head, Department of Chemistry, for providing laboratory facilities.
References
- Rao J.S. Sreenivasulu B. & Mogilaiah K.,Indian J. Chem., 34B, 734 (1995)
- Kidwai M., Bala R. & Kumar K., Indian J. Pharm. Sci., 57, 252 (1995)
- Hartmann H., Czerney P. & Liebsher J., Ger Pat., DD 204, 094, 1983; Chem. Abstr., 101, 7145 (1984)
- Feng X., Chen R. & Lin T., Youji Huxaxue, 14, 1994, 293; Chem. Abstr., 121, 205276 (1994)
- Troulman, H.D. & Long, L.M., J. Am. Chem Soc., 70, 3486 (1948)
- Coyne W.E., Medicinal Chemistry, Edited by Alfred Burger. Wiley Interscience, N.Y. (1970)
- Rawat T.R. & Srivastava S.D., Indian J. Chem., 37B, 91 (1998)
- Vogel, A.I. “A Textbook Of Practical Organic Chemistry”, 4th Edition., 651 (1978)
- Bernstein, H.I. & Rothstein, L.R., J. Am. Chem Soc., 66, 1886 (1944)
- Vogel, A.I. “A Textbook Of Practical Organic Chemistry”, 4th Edition., 652- 653 (1978)
- Bellamy, L. J., The Infrared Spectra of Complex Molecules, 2nd Edition, Methuen London, (1958)

This work is licensed under a Creative Commons Attribution 4.0 International License.





