Manuscript accepted on : 28-Mar-2019
Published online on: --
Plagiarism Check: Yes
Buffering Reduces Potassium Solubilising Potential of Selected Bacterial Isolates
Mahendra Vikram Singh Rajawat1,2 , Rajni Singh2
, Rajni Singh2 , Devendra Singh3
, Devendra Singh3 and Anil Kumar Saxena*1
and Anil Kumar Saxena*1
1ICAR-National Bureau of Agriculturally Important Microorganisms, Kushmaur, Maunath Bhanjan, Uttar Pradesh – 275103, India.
2Amity Institute of Microbial Biotechnology, Amity University, Noida, Uttar Pradesh – 201303, India.
3Dr. Rajendra Prasad Central Agricultural University Pusa, Samastipur, Bihar - 848125, India.
Corresponding Author E-mail: saxena461@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2716
ABSTRACT: Twenty three potassium solubilising bacteria (KSB) having potential to weather insoluble potassium bearing minerals were selected from 54 isolates obtained from the rhizosphere of different crop plants. The amount of K solubilised by different isolates in liquid medium ranged from 10.6±0.92 to 50.80±2.43 µg/mL. The isolates positive for solubilisation were categorized into weak, average and good solubiliser. Eleven isolates representing each category were further selected to study the effect of buffering of medium on K solubilisation. All the isolates exhibited the trait of K solubilisation both in the buffered and non-buffered media but with varying efficiency. In general for all the isolates, the amount of K released in the medium was low in buffered medium as compared to non-buffered medium. The buffering strength of medium diminished the effectiveness of KSB to release K from mineral and have no correlation with solubilisation in non-buffered medium (r = 0.89).
KEYWORDS: Aleksandrov Medium; Buffered and Non-Buffered Medium; Potassium Aluminosilicate; Potassium Solubilising Bacteria
Download this article as:| Copy the following to cite this article: Rajawat M. V. S, Singh R, Singh D, Saxena A. K. Buffering Reduces Potassium Solubilising Potential of Selected Bacterial Isolates. Biosci Biotech Res Asia 2019;16(1). |
| Copy the following to cite this URL: Rajawat M. V. S, Singh R, Singh D, Saxena A. K. Buffering Reduces Potassium Solubilising Potential of Selected Bacterial Isolates. Biosci Biotech Res Asia 2019;16(1). Available from: https://bit.ly/2WXv6tS |
Introduction
Potassium (K) is mainly found in soil locked in minerals like feldspar, mica, illite, muscovite which cannot be utilized by plant for their growth.1 There is very little amount of water soluble or available potassium in soil (0.03-3%) that is utilized by plants for their growth and other metabolic activities.2 Potassium is a major nutrient for plants that contributes in improvement of grain quality of crops.3-5 Potassium reacts with other elements like aluminium, silica, iron etc. in soil and forms an insoluble compound that is known as silicate minerals. Microbes occur in rhizosphere and function as intermediary agent to provide soluble form of potassium for plants. They mainly have capability to weather insoluble form of mineral to soluble form and enhance the accessibility of potassium to plants. These potential microbes in rhizosphere are known as potassium solubilisers and have capability to increase the availability of K in soil. Potassium solubilising potential has been found in several bacteria belonging to genus Bacillus, Pseudomonas, Paenibacillus, Arthrobacter,Azotobacter, Mesorhizobium and many more.6-12
Potassium solubilising bacteria leads to the weathering of potassium-bearing minerals by secreting different type of organic acids like oxalic acid, tartaric acid, gluconic acid, citric acid and release the potassium in surrounding environment which is utilized by plants.5,13-16 The mechanism for K solubilisation has parallel in P solubilisation by bacteria. Both the processes are predominantly executed by microorganisms through the production of different types of organic acids. There are few studies available wherein it has been reported that there is reduction in the amount of P solubilised when the medium for growth is buffered.17-19 Till now, most of the KSBs have been reported using non-buffered medium. Buffering capability of soil could hinder the solubilisation process as majority of the reports showed that lowering the pH of medium favours mineral weathering resulting in the release of nutrients.6,20,21 Hence it is worthwhile that parallel to the comparative studies on amount of P solubilised by microbes in buffered and non-buffered media, the study should be carried out to look for the influence of buffering of medium on different categories of K solubiliser which includes weak, average and good solubilisers.
Materials and Methodology
Bacterial Cultures
A total of 54 bacteria isolated from rhizospheric soils of different crop plants were obtained from culture collection, Division of Microbiology, ICAR- Indian Agricultural Research Institute, New Delhi. The cultures were revived, purified and stored at 4°C for further work.
Screening of Potassium Solubilising Potential
Bacterial isolates were grown in nutrient broth medium at 30°C for 16 hrs and 10 µl of each isolate was spot inoculated on Aleksandrov agar plates. Six cultures were spot inoculated on each plate and incubated at 30°C for 5 days. The composition of Aleksandrov medium (g/L) was: glucose 5.0, magnesium sulphate 0.5, ferric chloride 0.005, calcium carbonate 0.1, calcium phosphate 2.0, potassium bearing minerals 2.0. Potassium aluminosilicates were used as an insoluble potassium bearing minerals.7 The pH of the medium was adjusted to 7.2 by adding 1M NaOH. Cultures that showed halo zone around the colonies were selected and relative potassium solubilising ability was calculated by following formula.
Relative potassium solubilising ability = (Total zone size – colony size)/colony size
Aleksandrov broth medium containing potassium aluminosilicate as insoluble K source was inoculated with selected KSB (1% of freshly grown culture to 50 mL broth in triplicates) for 5 days and solubilised K in liquid medium was estimated through flame photometric method.22 Un-inoculated medium served as control.
Influence of Buffering on Potassium Solubilisation
The influence of buffering on potassium solubilising potential of bacterial isolates was carried out using Aleksandrov medium amended with 100mM Tris-Cl, pH 7.2. The influence of buffering on potassium solubilising potential was determined through measurement of halo zone surrounding the colonies of bacterial isolates. Quantitative estimation of solubilised potassium was also performed in both non-buffered and buffered medium through standard protocols.22 A solution of KCl (20, 30 and 40 ppm) was used as a standard for the analysis of available K.
Statistical Analysis
Obtained data were analysed statistically by using SPSS 16.0 statistical software. Mean value and standard error mean (SEM) between bacterial isolates and solubilisation under buffered and non-buffered condition. The mean values and SEM were calculated at 1% level of significance and represented as Mean ±SEM in the tables.
Results
Potassium Solubilising Bacteria
Among 54 bacterial isolates obtained, only 25 isolates showed the potential to solubilise insoluble potassium aluminosilicate mineral and formed distinguished halo zone around the colony (Fig. 1). Based on qualitative study, the relative potassium solubilising ability of isolates ranged from 1.12 to 1.69. The amount of K solubilised by different isolates in liquid medium ranged from 10.6±0.92 to 50.80±2.43 µg/mL, the highest value being achieved by KSB 7 (50.80±2.43 µg/mL) followed by KSB 5 (45.80±1.76). A significant reduction in pH of the medium was also observed during solubilisation study in liquid medium (Table 1). No halo zones around the colonies were detected for isolates KSB 3, KSB 4 and KSB 33, however these isolates were positive for K solubilisation in quantitative assay.
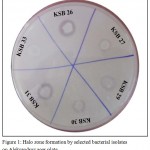 |
Figure 1: Halo zone formation by selected bacterial isolates on Aleksandrov agar plate.
|
Table 1: Screening of potassium solubilising isolates.
| Isolates | |||
| KSB 1 | 3.72 | 44.90±1.35 | 1.17 |
| KSB 2 | 4.12 | 34.50±0.40 | 1.69 |
| KSB 3 | 5.36 | 14.90±1.87 | ND |
| KSB 4 | 4.43 | 13.60±0.56 | ND |
| KSB 5 | 3.56 | 45.80±1.76 | 1.14 |
| KSB 6 | 3.83 | 40.40±2.01 | 1.12 |
| KSB 7 | 3.46 | 50.80±2.43 | 1.17 |
| KSB 12 | 4.34 | 22.00±0.98 | 1.29 |
| KSB 13 | 4.67 | 22.40±1.11 | 1.14 |
| KSB 14 | 5.24 | 21.60±2.03 | 1.20 |
| KSB 15 | 4.93 | 24.70±1.90 | 1.20 |
| KSB 16 | 5.23 | 18.90±2.66 | 1.24 |
| KSB 19 | 5.46 | 14.20±1.30 | 1.32 |
| KSB 20 | 4.76 | 26.30±2.17 | 1.20 |
| KSB 22 | 5.52 | 15.60±1.06 | 1.19 |
| KSB 23 | 3.23 | 40.20±1.51 | 1.57 |
| KSB 24 | 5.14 | 17.90±1.41 | 1.13 |
| KSB 25 | 3.76 | 18.90±1.85 | 1.30 |
| KSB 26 | 5.26 | 15.70±0.52 | 1.81 |
| KSB 27 | 5.20 | 17.90±1.30 | 1.33 |
| KSB 28 | 4.56 | 14.20±1.15 | 1.64 |
| KSB 29 | 4.11 | 18.70±1.35 | 1.24 |
| KSB 30 | 3.90 | 21.70±1.21 | 1.14 |
| KSB 31 | 4.25 | 15.10±1.10 | 1.54 |
| KSB 33 | 4.86 | 10.60±0.92 | ND |
*data showed the mean value and standard error mean of triplicates.
Potassium Solubilising Efficiency Under Buffered Condition
Based on quantitative estimation of solubilised potassium, the isolates positive for solubilisation were categorized into weak, average and good solubiliser. Eleven isolates representing each category were further selected to study the effect of buffering of medium on K solubilisation. It included 5 good (KSB 1, KSB 2, KSB 5, KSB 6 and KSB 7), 2 average (KSB 12 and KSB 30) and 4 weak (KSB 26, KSB 27, KSB 29 and KSB 31) K solubilisers. A differential response among the isolates was observed irrespective of their strength of solubilisation. Moreover the results of qualitative (halo zone formation) and quantitative analysis (flame photometer) also showed variations. Isolates KSB 2, KSB 5, KSB 7, KSB 12, KSB 27, KSB 29 and KSB 30 exhibited zones of solubilisation in non-buffered medium but failed to do so in buffered medium (Fig. 2, Table 2). However in quantitative assays, all these cultures were able to solubilise K in broth medium. These results indicate that size of zone of solubilisation is not a good criterion to determine the efficiency of isolate for K solubilisation. Therefore the further comparisons were made only for quantitative estimations. In general, for all the isolates there was reduction in the amount of K solubilised in buffered medium as compared to non-buffered medium. Buffering had a greater influence on the K solubilising capacity of isolates KSB 1, KSB 2, KSB 5, KSB 7 and KSB 29. Other isolates also showed reduction in the amount of K solubilised in buffered medium, but was to a lesser extent.
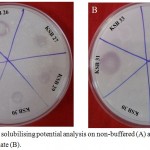 |
Figure 2: Potassium solubilising potential analysis on non-buffered (A) and buffered Aleksandrov agar plate (B).
|
Table 2: Influence of buffering on selected isolates of potassium solubilising bacteria.
| K-source | Buffer | Bacterial isolates | Size of halo zone (mm) | pH | Amount of K solubilised (µg/mL)* |
| Potassium aluminosilicate | – | KSB 1 | 3.17±0.29 | 3.32 | 47.30±1.65 |
| + | KSB 1 | 0.75±0.25 | 3.65 | 38.20±1.05 | |
| – | KSB 2 | 0.77±0.25 | 3.84 | 33.80±0.92 | |
| + | KSB 2 | ND | 4.45 | 19.60±1.51 | |
| – | KSB 5 | 0.67±0.29 | 3.15 | 46.70±2.19 | |
| + | KSB 5 | ND | 3.30 | 22.40±0.53 | |
| – | KSB 6 | 2.08±0.38 | 4.12 | 42.20±1.20 | |
| + | KSB 6 | 1.03±0.45 | 4.35 | 34.80±1.78 | |
| – | KSB 7 | 0.75±0.25 | 3.86 | 48.20±2.63 | |
| + | KSB 7 | ND | 4.23 | 28.30±1.15 | |
| – | KSB 12 | 2.00±0.25 | 3.78 | 22.80±0.61 | |
| + | KSB 12 | ND | 3.98 | 16.30±1.08 | |
| – | KSB 26 | 5.25±0.43 | 4.65 | 16.30±1.51 | |
| + | KSB 26 | 5.45±0.18 | 4.85 | 12.60±0.70 | |
| – | KSB 27 | 0.67±0.29 | 5.50 | 18.40±0.95 | |
| + | KSB 27 | ND | 5.58 | 12.70±0.98 | |
| – | KSB 29 | 0.83±0.25 | 3.89 | 18.10±0.50 | |
| + | KSB 29 | ND | 3.95 | 9.80±0.35 | |
| – | KSB 30 | 1.58±0.52 | 4.35 | 22.60±0.89 | |
| + | KSB 30 | ND | 4.73 | 17.20±0.95 | |
| – | KSB 31 | 4.08±0.38 | 4.11 | 16.20±1.05 | |
| + | KSB 31 | 4.50±0.67 | 4.52 | 9.40±0.63 |
* Data showed the mean value±standard error mean of triplicates.
Relationship of Potassium Solubilising Efficiency Between Buffered and Non-Buffered Medium
Data obtained with quantitative estimation of solubilised potassium in buffered and non-buffered medium has no significant correlation (r = 0.89). It showed that potassium solubilising efficiency of isolates differs in both buffered and non-buffered condition. There is no linearity (R2 = 0.789) among the values obtained from both condition (Fig. 3).
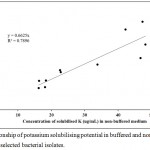 |
Figure 3: Relationship of potassium solubilising potential in buffered and non-buffered medium among selected bacterial isolates.
|
Discussion
Many of the plant nutrients like phosphorus, potassium and zinc are not present in the available form and are fixed in the soil. As a consequence, these nutrients are not available to the plants and thus becomes limiting for the growth and yield of crop plants. In soils there are many bacteria, fungi and archaea that have the ability to release these nutrients from the complex sources and hence make it available to the plants.5,9,23 The mechanism of P solubilisation is well studied as compared to K and Zn solubilisation. Different mechanisms have been proposed for release of P from complexes and mainly include production of organic acids, protons, hydroxyl ions, siderophores and CO2. In addition there are also reports of production of inorganic acids like HCl and HNO3.24 Among all these, organic acid production accompanied by reduction in pH is considered to be the chief mechanism. There are reports of inoculation failure of efficient strains of P solubilising bacteria in soils. It is believed that soils have a good buffering capacity and hence reduce the efficiency of P solubilising bacteria to release P from the complex.18 Soils with Ca-P as a major phosphorus source also have a high buffering capacity.25
Likewise, K solubilisation is also reported to be the production of different types of organic acids by microorganisms.5,7,11,12,15,26,28 Different organic acids like glycolic acid, succinic acid, citric acid, tartaric acids, 2-ketogluconic acid, oxalic acid, gluconic acid, malic acid, propionic acid, and fumaric acid have been implicated in K solubilisation.12,13,15,29 Acidification of the medium due to production of acids plays an important role in solubilisation of nutrient ions like P. K and Zn. However, acidification may not be the sole mechanism of solubilisation, as no correlation has been reported between the ability to reduce pH and P solubilisation.30 The chelating ability of organic acids is also important, as it has been shown that the addition of 0.05M EDTA to the medium has the same solubilising effect as inoculation with Penicillium bilaii.31 The inability of P solubilising Rhizobium strains to solubilise P due to the addition of NaOH indicates the importance of acidification in P solubilisation.32 Likewise buffering of the medium by addition of 100mM Tris HCl also reduced the efficiency of strains to solubilise P.18,33,34 Gyaneshwar et al., (1998) also observed reduction in P solubilising ability of Bacillus coagulans and Citrobacter koseri in buffered medium.17
There are no reports available for the performance of strains in buffered vis a vis non buffered medium. In the present study 11 isolates varying in their ability to solubilise K in broth were evaluated for K solubilisation in both buffered and non-buffered medium. In general, all the isolates showed reduction in K solubilising activity to varying levels in buffered medium as compared to non-buffered medium. It further confirms that production of acids and acidification of medium is the major mechanism of K solubilisation by bacteria.
Conclusion
The results reveal that there is a reduction in the potential of bacterial isolates to release potassium from aluminum silicates in buffered medium as compared to non-buffered medium. It indicates that production of organic acids and lowering of pH (acidification) is the major mechanism for K solubilisation by microorgansisms. Moreover, potassium solubilising efficiency should be analysed in buffered medium as it might be helpful to identify more potential potassium solubilisers.
Acknowledgements
The authors are grateful to the Division of Microbiology, ICAR-Indian Agricultural Research Institute, New Delhi and Department of Biotechnology (DBT), Ministry of Science and Technology for providing the facilities and financial support, to undertake the investigations.
Conflict of Interest
There is no conflict of interest.
References
- Buchholz D. D., Brown J. Potassium in Missouri soils. 1993.
- Sparks D., Huang P. Physical chemistry of soil potassium. Potassium in agriculture (potassiuminagri). 1985;201-276.
- Biswas D., Basak B. Mobilization of potassium from waste mica by potassium-solubilizing bacteria (Bacillus mucilaginosus) as influenced by temperature and incubation period under in vitro laboratory conditions. Agrochimica. 2014;58(4):309-320.
- Singh G., Biswas D., Marwaha T. Mobilization of potassium from waste mica by plant growth promoting rhizobacteria and its assimilation by maize (Zea mays) and wheat (Triticum aestivum L.): a hydroponics study under phytotron growth chamber. J. Plant Nutr. 2010;33(8):1236-1251.
- Zhang C., Kong F. Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl. Soil Ecol. 2014;82:18-25.
- Ae N., Arihara J., Okada K., Yoshihara T., Johansen C. Phosphorus uptake by pigeon pea and its role in cropping systems of the Indian subcontinent. Science. 1990;248(4954):477-480.
- Hu X., Chen J., Guo J. Two phosphate-and potassium-solubilizing bacteria isolated from Tianmu Mountain, Zhejiang, China. World J. Microb. Biot. 2006;22(9):983-990.
- Huang Z., He L. y., Sheng X. f., He Z. Weathering of potash feldspar by Bacillus sp. L11. Acta Microbiol. Sin. 2013;53(11):1172-1178.
- Rajawat M. V. S., Singh S., Saxena A. K. A new spectrophotometric method for quantification of potassium solubilized by bacterial cultures. Indian J. Exp. Biol. 2014;52(3):261-266.
- Sarikhani M. R., Khoshru B., Oustan S. Efficiency of Some Bacterial Strains in Potassium Release from Mica and Phosphate Solubilization under In Vitro Conditions. Geomicrobiol. J. 2016;33(9):832-838.
- Sheng X. F., Zhao F., He L. Y., Qiu G., Chen L. Isolation and characterization of silicate mineral-solubilizing Bacillus globisporus Q12 from the surfaces of weathered feldspar. Can. J. Microbiol. 2008;54(12):1064-1068.
- Xiao Y., Wang X., Chen W., Huang Q. Isolation and identification of three potassium-solubilizing bacteria from rape rhizospheric soil and their effects on ryegrass. Geomicrobiol. J. 2017;34(10):873-880.
- Zarjani J. K., Aliasgharzad N., Oustan S., Emadi M., Ahmadi A. Isolation and characterization of potassium solubilizing bacteria in some Iranian soils. Arch. Agron. Soil Sci. 2013;59(12):1713-1723.
- Meena V. S., Maurya B., Verma J. P. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol. Res. 2014;169(5-6):337-347.
- Meena V. S., Maurya B. R., Verma J. P., Aeron A., Kumar A., Kim K., Bajpai V. K. Potassium solubilizing rhizobacteria (KSR): isolation, identification and K-release dynamics from waste mica. Ecol. Eng. 2015;81:340-347.
- Parmar P., Sindhu S. Potassium solubilization by rhizosphere bacteria: influence of nutritional and environmental conditions. J. Microbiol. Res. 2013;3(1):25-31.
- Gyaneshwar P., Kumar G. N., Parekh L. Effect of buffering on the phosphate-solubilizing ability of microorganisms. World J. Microb. Biot. 1998;14(5):669-673.
- Joseph S., Jisha M. Buffering reduces phosphate solubilizing ability of selected strains of bacteria. World J. Agric. Sci. 2009;5 (1):135-137.
- Nobahar A., Sarikhani M. R., Chalabianlou N. Buffering capacity affects phosphorous solubilization assays in rhizobacteria. Rhizosphere. 2017;4:119-125.
- Hariprasad P., Niranjana S. Isolation and characterization of phosphate solubilizing rhizobacteria to improve plant health of tomato. Plant Soil. 2009;316(1-2):13-24.
- Maliha R., Samina K., Najma A., Sadia A., Farooq L. Organic acids production and phosphate solubilization by phosphate solubilizing microorganisms under in vitro conditions. Pak. J. Biol. Sci. 2004;7:187-196.
- Jackson M. Estimation of Potassium Content: Soil Chemical Analysis. Printer Hall, New Delhi (India). 1973.
- Yadav A. N., Sharma D., Gulati S., Singh S., Dey R., Pal K. K., Kaushik R., Saxena A. K. Haloarchaea endowed with phosphorus solubilization attribute implicated in phosphorus cycle. Sci. Rep.-UK. 2015;5:12293.
- Alori E. T., Glick B. R., Babalola O. O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017;8:971.
- Ae N., Arihara J., Okada K. Phosphorus response of chickpea and evaluation of phosphorus availability in Indian Alfisols and Vertisols. Phosphorus nutrition of grain legumes in the semi-arid tropics (Johansen C., Lee K. K and Sahrawat K. L., eds) Patancheru. 1991;502(324):33-41.
- Barker W., Welch S., Chu S., Banfield J. Experimental observations of the effects of bacteria on aluminosilicate weathering. Am. Mineral. 1998;83(11):1551-1563.
- Bennett P., Choi W., Rogera J. Microbial destruction of feldspars. Mineral Manag. 1998;8(62A):149-150.
- Zhang A. m., Zhao G. y., Gao T. g., Wang W., Li J., Zhang S. f., Zhu B. c. Solubilization of insoluble potassium and phosphate by Paenibacillus kribensis CX-7: a soil microorganism with biological control potential. Afr. J. Microbiol. Res. 2013;7(1):41-47.
- Prajapati K., Modi H. Isolation and characterization of potassium solubilizing bacteria from ceramic industry soil. CIBTech. J. Microbiol. 2012;1(2-3):8-14.
- Subba Rao N. Phosphate solubilization by soil microorganisms. Advances in agricultural microbiology/edited by NS Subba Rao. 1982.
- Kucey R. Effect of Penicillium bilaji on the solubility and uptake of P and micronutrients from soil by wheat. Can. J. Soil Sci. 1988;68(2):261-270.
- Halder A., Chakrabartty P. Solubilization of inorganic phosphate by Rhizobium. Folia Microbiol. 1993;38(4):325-330.
- Cunningham J. E., Kuiack C. Production of citric and oxalic acids and solubilization of calcium phosphate by Penicillium bilaii. Appl. Environ. Microbiol. 1992;58(5):1451-1458.
- Halder A., Mishra A., Chakrabarty P. Solubilization of inorganic phosphates by Bradyrhizobium. Indian J. Exp. Biol. 1991;29(1):28-31.

This work is licensed under a Creative Commons Attribution 4.0 International License.





