Manuscript accepted on : 9-December-2018
Published online on: 24-12-2018
Plagiarism Check: Yes
P. Shiny Arokiamary, A. Vinoth Alphonse and R. Ravindhran
T. A. L. Samy Unit for Plant Tissue Culture and Molecular Biology, Department of Plant Biology and Biotechnology, Loyola College (Autonomous), Nungambakkam, Chennai – 600 034, Tamil Nadu, India.
Corresponding Author E-mail: raviloyola1998@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2707
ABSTRACT: Couroupita guianensis Aubl. popularly known as cannonball tree is widely distributed in the tropical regions. The tree parts are commonly used to treat wounds and tumors. Leaves, flowers, and fruits contain active phytochemicals with significant biological activity. In the recent years, destruction of natural habitats by mankind has reduced its distribution. Natural propagation of C. guianensis by seeds is greatly hindered by poor seed germination and viability. Therefore, the present study was undertaken to optimize the conditions for in vitro embryo germination and to investigate the seed storage behaviour. Mature seeds inoculated on MS basal medium germinated within 10 d with a frequency of 61.6%. Supplementation of plant growth regulators (PGRs) to MS medium improved the embryo germination frequency (100%). Seedlings with highest shoot length (8.10±0.11 cm) and root length (6.27±0.14 cm) were produced in MS medium supplemented with 1.0 mg/l kinetin and 0.1 mg/l indole-3-butyric acid. Among different strength liquid MS basal salts, quarter-strength produced a greater number of secondary roots (8.00±0.28) with average root length of 17.83±0.58 cm. Seed storage behaviour studies clearly proved the recalcitrant nature as only freshly harvested mature seeds retained the germination potential upon storage at 15 ºC for up to 45 d. Desiccation of seeds on exposure to air-dry storage resulted in rapid deterioration of germination. Pre-conditioning of germinated seedlings in liquid MS basal salts was required for their survival under field conditions. Plantlets with well-developed roots were successfully acclimatized to the field with 100% survivability. This protocol facilitates conservation, sustainable utilization and re-introduction of C. guianensis into its natural habitats.
KEYWORDS: Couroupita Guianensis; Mature Seeds; Plant Growth Regulators; Seed Viability; Secondary Rooting
Download this article as:| Copy the following to cite this article: Arokiamary P. S, Alphonse A. V, Ravindhran R. Factors Influencing in Vitro Germination and Seed Storage Behavior of Couroupita Guianensis Aubl – A Useful Tropical Tree Species. Biosci Biotech Res Asia 2018;15(4). |
| Copy the following to cite this URL: Arokiamary P. S, Alphonse A. V, Ravindhran R. Factors Influencing in Vitro Germination and Seed Storage Behavior of Couroupita Guianensis Aubl – A Useful Tropical Tree Species. Biosci Biotech Res Asia 2018;15(4). Available from: https://www.biotech-asia.org/?p=32214 |
Introduction
Couroupita guianensis is a medicinally valuable tree used in folk medicines and belongs to the family Lecythidaceae. The tree commonly known as cannonball tree is a large, deciduous, tropical, soft-wooded tree, native to South India, Malaysia, Thailand, Singapore, Sri Lanka, and Bangladesh. The leaves are alternate, oblong, up to 20 cm long, entire to slightly serrate and hairy on the veins beneath.1 The inflorescence is racemose, arising from the trunk and other large branches. Flowers are reddish with a yellow tinge on the outside, fragrant, with stamens borne on an overarching androphore. Fruit is a large, reddish-brown, globose, with a woody capsule, resembling big rusty the cannonballs hanging in clusters on a string2 (Fig. 3a). The mature fruit is 24 cm in diameter, weighing around 1.5 kg. Fruits contain 80-300 seeds buried within the pulpy mesocarp. The seed coat is covered by exotestal hairs, a specialized adaptation for endozoochory.3 In folk medicine, the tree parts are used to treat hypertension, tumours, pain, inflammation, cold, stomach ache, skin diseases, malaria, wounds and toothache.4 The fruit pulp is used to treat the infected skin of mange dogs5 and the flowers are used as a cure for the intestinal gas formation and stomach ache.6 The leaves are used in herbal hand wash formulations and the aliphatic triterpene is used as an anti-depressant in rats.7 The phenolic compounds are active in curing the kidney and stomach problems and act as anti-inflammatory and anti-diabetic agents.8,9
Sustainable utilization of C. guianensis in traditional medicine is highly dependent on the successful propagation of this tree species. Under natural conditions, seeds of C. guianensis fail to germinate, as the fruit pulp is highly susceptible to bacterial and fungal infestation and eaten by peccaries, pigs and monkeys. Very few seeds that escape the degradation from their digestive enzymes, because of the presence of hairy seed coat, germinate under suitable conditions.10 But the seedlings die soon after emergence due to rapid drying of the primary roots. In vitro seed germination is thus an economically viable option as it circumvents the environmental constraints and offers superior quality plantlets over greenhouse raised plants.11 In vitro germination of zygotic embryo produces various explants well-suited for the conservation of medicinally important tree species in genebanks.12,13 Nevertheless, the knowledge on seed storage behaviour of target species is critical to determine the seed storage as a viable option for germplasm conservation.
Seed desiccation sensitivity is explicitly mentioned in plant regeneration ecology reported by.14 This is perhaps a consequence of the trait conferring few obvious problems in nature; plants with drying sensitive seeds continue to produce seedlings. Desiccation sensitive seeds rapidly lose viability on drying and cannot be stored in conventional seed bank facilities. The difficulties presented by these seeds have resulted in their being termed ‘recalcitrant’.15 The seed moisture content below which viability is lost varies significantly between species, but is generally above 20%.16 In addition, the level of drying that recalcitrant species can tolerate can vary due to a range of other factors, including seasonal differences in the extent of pre-shedding (maturation) drying17 and environmental features such as postharvest and drying conditions.18
The present research work was undertaken to understand the role of various factors like moisture content and germinability of mature and immature seeds, desiccation effects on germination of C. guianensis seeds, Storage temperature effects on germination of C. guianensis seeds, Longevity of C. guianensis seeds stored at different moisture conditions are affecting the seed storage behaviour. In this investigation, we also report the establishment of healthy seedlings of C. guianensis by the application of plant growth regulators to the germination medium. The optimized conditions described in this study would accelerate the establishment of healthy seedlings of C. guianensis by in vitro germination. This protocol facilitates replanting of C. guianensis into its natural habitats.
Materials and methods
Plant material and surface sterilization
Couroupita guianensis fruits were collected from Loyola College hostel, Chennai, India (13°03’41.7″N 80°13’57.5″E). Mature and healthy seeds were harvested from the pulp of ripened fruits after removing the outer shell mechanically. The seeds were washed under running tap water for 15 min to remove the pulp debris and dried overnight at room temperature. Steps involved in surface sterilization process (Fig. 1). The outer layer of the seed coat with exotestal hairs was removed manually and seeds were surface sterilized using 1% (v/v) sodium hypochlorite (available Chlorine 4% w/v approx, Qualigens, Mumbai, India) containing 2-4 drops of Tween-20 per 100 ml for 20 min. The seeds were then rinsed with autoclaved double distilled water for 6 to 8 times under laminar air flow cabinet. After surface sterilization, exotestal hairs with seed coat (~0.4 mm thick) and thin endosperm were removed with a sterile blade, embryos were blot dried on a sterile filter paper and used for germination.
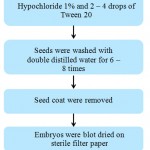 |
Figure 1: Steps involved the surface sterilization of the explant |
Effect of plant growth regulators on in vitro growth of C. guianensis embryos
Excised embroys were germinated on Murashige and Skoog (MS)19 medium supplemented with different concentrations of plant growth regulators (PGRs), such as 6-benzylaminopurine (BAP) and kinetin (KIN) (1.0, 2.0 mg/l) individually, or in combination with 0.1-0.5 mg/l α-naphthalene acetic acid (NAA), indole-3-acetic acid (IAA), and indole-3-butyric acid (IBA) (HiMedia, Mumbai, India). The seed germination medium consisted of 3% sucrose (Merck, Bangalore, India) as the carbon source and 8 g/l agar (HiMedia, Mumbai, India) as the gelling agent. The pH of the medium was adjusted to 5.8±0.02 using 1.0 N NaOH or 1.0 N HCl before adding the agar. Cultures were maintained in test tubes (25 mm-diameter × 150 mm-height) (Borosil, Chennai, India), and incubated at 25±1 °C in darkness for 3 d. After 3 d, the cultures were transferred to 16/8- h (day/night) photoperiod with a light intensity of 50 µmol m-2 s-1 supplied with cool-white fluorescent lamps (Philips, Chennai, India).
Effect of liquid MS basal salts on secondary root induction and acclimatization
Healthy seedlings (2 wk old, 5-6 cm in height) established from mature embryos (freshly harvested) germinated on MS medium containing 1.0 mg/l KIN and 0.1 mg/l IBA were carefully removed from the culture tubes, washed with sterile water to remove the agar media, blot dried on filter paper and placed in different strength (quarter-, half-, and full-strength) liquid MS basal salts without shaking for the induction of secondary roots. After 10 d, plantlets (15-18 cm in height) with well-developed secondary roots were transferred to paper cups (6-cm diameter) filled with soil mixture containing sterilized garden soil and red soil and sand (1:1:1, v/v/v). These plants were maintained in the culture room at 25±1 ºC and 16/8-h (day/night) photoperiod with a light intensity of 50 µmol m-2 s-1 for 2 d and then transferred to HDPE grow bags (30-cm wide×35-cm tall; SK Organic Farms, Chennai, India) containing mixture of garden soil, red soil and sand (1:1:1, v/v/v) and grow to under greenhouse conditions. Acclimatized plants with six to ten new leaves were transferred to clay pots and plants about 60 cm in height were planted in soil.
Moisture content and germinability of mature and immature seeds
Seed storage hehaviour of C. guianensis was determined by four independent experiments (Fig. 2).
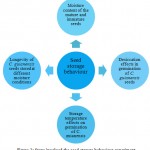 |
Figure 2: Steps involved the seed storage behaviour experiment |
Freshly harvested seeds of different weight [(mature seeds having dense exotestal hairs and well-developed embryo; immature seeds covered by thin exotestal hairs and poorly-developed embryos)] and initial moisture content (IMC) were tested for seed germination potential. The initial moisture content (IMC) of the seeds was calculated using the formula, IMC = [Fresh weight – Dry weight / Fresh weight] × 100. The dry weight (DW) of the seeds was determined after dehydration in hot air oven at 130°C for 4 h.
Desiccation effects on germination of C. guianensis seeds
Mature seeds were subjected to dehydration by rapid and slow drying process until a desired moisture content (DMC) (in a fresh weight basis) reached 20%. Rapid drying was achieved by incubating the seeds in incubator (EPS Biosolutions, Chennai) at 37°C while slow drying was performed at room temperature (28°C). Experiment 3: Mature seeds were dehydrated by rapid drying process to DMC ranging from 32 to 4% in decreasing steps of 4%. DMC of seed lots was measured by indirect evaluation of seed weight after rapid dehydration for certain time period using the formula, Weight of seed (g) at DMC% = [(100-IMC%)/(100-DMC%)] × initial seed weight (seeds washed under running tap water were blot dried on tissue paper and weighted immediately) (g).
Storage temperature effects on germination of C. guianensis seeds
Mature seeds with 16% DMC, obtained by rapid dehydration, were stored at different temperatures (-20, 0, 4, 10, 15, 25, and 30°C) for 7 d to optimize the suitable storage temperature.
Longevity of C. guianensis seeds stored at different moisture conditions
Seed viability was tested by germination of mature seeds (freshly harvested) stored for 90 d at 15°C. The interaction between seed moisture content and storage temperature, as just one temperature has been used. The only difference between this experiment and experiment 3 is that seeds were stored for 2 weeks at 15°C after drying to diverse moisture contents. Seed germination was determined by measuring the growth parameters of the seedlings germinated from seeds of different DMC stored at 15°C for 2 wk. In all the above experiments, the seeds subjected to various physical conditions were germinated on MS medium containing 1.0 mg/l KIN and 0.1 mg/l IBA. Cultures were maintained in the same conditions as described above for 2 wk.
Statistical Analysis
Treatment and control replicates for all the experiments followed complete randomized design and each experiment was repeated three times. To determine the role of PGRs and seed storage behaviour on in vitro embryos germination, three seed lots were used for each treatment with 10 uniform seeds per lot. For secondary root induction, 30 individual seedlings were used for each treatment. One-way analysis of variance (ANOVA) was performed and the data is presented as mean ± standard error. Duncan’s multiple range test at 5% probability level was performed using IBM SPSS statistics version 19.0 to detect the significant differences among the treatment means.
Results and Discussion
Effect of plant growth regulators on in vitro embryo of Couroupita guianensis
Embryo germination, in general, is characterized by cell division and elongation in the embryonal axis, i.e. radicle and plumule.20 Plant growth regulators (PGRs) including cytokinins, auxins, gibberellric acid and abscisic acid play a significant role in germination by breaking the seed dormancy.21,22 Seeds inoculated on MS medium (Fig. 3b) containing PGRs germinated uniformly by radicle emergence within 3 days under darkness (Fig. 3c). After 3 days, transfer of culture to 16/8-h (day/night) photoperiod resulted in hypocotyl elongation and the opening of cotyledonary leaves to expose the shoot apex (Fig. 3d-e). The supplementation of PGRs to MS basal medium improved the embryo germination frequency to greater than 85% in all the combinations tested (Table 1). The highest embryo germination frequency (100%) was observed in MS medium supplemented with 1.0 mg/l BAP+0.5 mg/l IAA, 1.0 mg/l BAP+0.1 mg/l NAA, 1.0 mg/l KIN, and 1.0 mg/l KIN+0.1 mg/l IBA (Fig. 3f). BAP or KIN (1.0 mg/l) when used individually produced the shoot and root length of 6.05±0.29 cm, 4.14±0.35 cm and 4.22±0.21 cm, 4.96±0.18 cm, respectively. In concurrence with our results, addition of 0.5 mg/l BAP to MS medium produced 90% seed germination in Shorea tumbuggaia.23 Combination of IBA with BAP or KIN increased the shoot length and root length compared to IAA or NAA. The highest shoot length (8.10±0.11 cm) and root length (6.27±0.14 cm) was recorded in MS medium supplemented with 1.0 mg/l KIN+0.1 mg/l IBA. Root induction from in vitro regenerated plantlets of majority of tree species was achieved using IBA [Azadirachta indica]24; [Dalbergia retusa]25; [Eucalyptus globulus]26; [Sapium sebiferum]27; [Garcinia indica]28; [Pinus thunbergii].29 Contrasting our studies, reports indicate callus formation from seeds and reduced germination percentage in MS medium supplemented with auxins.13, 30 The embryo has been used for different moisture content, temperature and storage behavior study shows no germination, because of the presence of phenolic compounds. Embryo color was changed from yellow to brown. Likewise, in all the subsequent experiments were used for seeds.
 |
Figure 3: In vitro seed germination of Couroupita guianensis.
|
(a) Tree at Loyola College hostel garden, enlarged at the top right corner is the mature fruit; (b) mature seed inoculated on MS medium; (c) germination of mature seed on MS medium after 3 d; (d) Elongation of hypocotyl after 7 d, arrow indicates hypocotyl; (e) Opening of cotyledonary leaves in germinated seedlings after 10 d, arrow indicates shoot apex; (f) Germination of mature seeds in MS medium supplemented with PGRs; (g) Immature seed inoculated on MS medium; (h) Crinkled cotyledonary leaves of germinated seedlings from immature seed; (i) Healthy seedlings raised from freshly harvested mature seeds and after one month of storage at 4°C; (j) Hardening of seedlings in liquid MS basal salts; (k) Secondary root formation in germinated seedlings hardened using tap water and different strength liquid MS basal salts; (l) Acclimatization of 20-d-old seedlings transferred to plastic cups containing soil mixture [garden soil and red soil (1:1, v/v)]; (m) 45-d-old plantlets maintained in HDPE grow bags under greenhouse conditions; (n) 60-d-old plant maintained in clay pots; (o) 180-d-old plant in Loyola College garden. Bars= 5 mm (b-h); 10 mm (i-n)
Table 1: Effect of different concentrations and combinations of PGRs on in vitro seed germination of Couroupita guianensis
| PGRs (mg/l) | Seed germination frequency (%) | Shoot length (cm)#
|
Root length (cm)#
|
||||
| BAP | KIN | IBA | IAA | NAA | |||
| – | – | – | – | – | 61.66 | 4.68±0.17hi | 2.95±0.08j |
| 1.0 | – | – | – | 88 | 6.05±0.29c | 4.14±0.35h | |
| 1.0 | – | 0.1 | – | – | 93 | 5.47±0.64e | 5.19±0.24d |
| 1.0 | – | 0.5 | – | – | 95 | 5.49±0.09e | 4.99±0.30e |
| 2.0 | – | 0.1 | – | – | 91.33 | 5.61±0.34d | 6.27±0.05a |
| 1.0 | – | – | 0.1 | – | 92 | 3.94±0.30j | 4.61±0.10fg |
| 1.0 | – | – | 0.5 | – | 100 | 5.66±0.04d | 4.88±0.18ef |
| 2.0 | – | – | 0.1 | – | 94.33 | 5.16±0.04g | 4.66±0.12fg |
| 1.0 | – | – | – | 0.1 | 100 | 5.33±0.04ef | 4.55±0.12fg |
| 1.0 | – | – | – | 0.5 | 95.66 | 5.49±0.12e | 5.82±0.16c |
| 2.0 | – | – | – | 0.1 | 97.33 | 5.16±0.16g | 4.83±0.19ef |
| – | 1.0 | – | – | – | 100 | 4.22±0.21hi | 4.96±0.18e |
| – | 1.0 | 0.1 | – | – | 100 | 8.10±0.11a | 6.27±0.14a |
| – | 1.0 | 0.5 | – | – | 98.66 | 6.55±0.15b | 5.22±0.07d |
| – | 2.0 | 0.1 | – | – | 98 | 6.16±0.26c | 4.64±0.14fg |
| – | 1.0 | – | 0.1 | – | 98.66 | 5.44±0.49e | 6.11±0.30b |
| – | 1.0 | – | 0.5 | – | 98.66 | 4.99±0.41h | 4.99±0.31e |
| – | 2.0 | – | 0.1 | – | 97.33 | 4.38±0.23hi | 3.83±0.12i |
| – | 1.0 | – | – | 0.1 | 97.66 | 4.94±0.20h | 5.05±0.22d |
| – | 1.0 | – | – | 0.5 | 97 | 4.72±0.15hi | 3.82±0.21i |
| – | 2.0 | – | – | 0.1 | 99 | 5.38±0.32ef | 4.05±0.14h |
#Values represent mean ±SE. Different letters indicate significant difference among the treatment means analyzed using Duncan’s multiple range test (P<0.05).
Effect of liquid MS basal salts on secondary root induction and acclimatization
Root formation is a vital factor for the survival of seedlings upon transplantation to field conditions.31 Healthy seedlings grown on MS medium supplemented with PGRs did not survive transplantation to field conditions when transferred directly to soil mixture [garden soil and red soil (1:1, v/v)]. The primary roots formed in agar medium dried rapidly in soil mixture resulting in the death of the seedlings. This is because; in vitro roots are generally hypertrophic, lack root hairs and have poor vascular connections with the shoot resulting in restricted water movement to the shoots.32-34 In our study, additional hardening procedure was necessary for transfer of seedlings to the field. Hence, seedlings from agar medium were transferred to different strength (quarter-, half-, and full-strength) liquid MS basal salts to facilitate secondary root formation (Fig. 6). The plantlets transferred to liquid medium were maintained under culture room conditions at 25°C under 16/8-h (day/night) photoperiod for 7 d. The root portion was covered with aluminium foil to maintain darkness (Fig. 3j). Liquid MS basal salts facilitated secondary root initiation within 3 d with highest number of roots (8.00±0.28) and root length (17.83±0.58 cm) in quarter-strength (Fig.3k). In addition, elongation of shoot was observed in all the strengths and highest shoot length (21.16±0.30 cm) was recorded in quarter-strength liquid MS basal salts. Plantlets with secondary roots were successfully hardened to field conditions with survivability of 100% (Fig. 3o).
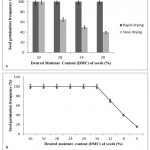 |
Figure 4: Effect of drying process and seed moisture content on in vitro germination of C. guianensis.
|
Germination potential of seeds (a) subjected to rapid and slow drying process; (b) rapid dehydrated to different desired moisture content (DMC, %). Data recorded after 10 d of culture on MS medium containing 1.0 mg/l KIN and 0.1 mg/l IBA
Moisture content and germinability of mature and immature seeds, Desiccation effects on germination of C. guianensis seeds, Storage temperature effects on germination of C. guianensis seeds, Longevity of C. guianensis seeds stored at different moisture conditions
Seeds are vital genetic resources for long-term conservation of tree species. Seeds of woody perennials display wide variations in their seed storage behaviour, thus classified into three types namely orthodox, intermediate and recalcitrant based on the moisture content and storage temperature. The intermediate and recalcitrant seeds are further subdivided into tropical and temperate origin.16 Couroupita guianensis is a tropical, recalcitrant tree species with poor seed viability.35 Seed storage behaviour of trees is generally influenced by various factors such as moisture content and germinability of mature and immature seeds, desiccation effects on germination of C. guianensis seeds, storage temperature effects on germination of C. guianensis seeds, longevity of C. guianensis seeds stored at different moisture conditions. Therefore, optimization of all these parameters is essential for genetic conservation of C. guianensis.
Seed maturity plays a vital role in seedling establishment as the seeds attain desiccation tolerance on maturity. Identification of mature fruits of C. guianensis was quite difficult as they possessed hard and fibrous pericarp. Therefore, fruits of larger size were preferred to extract the mature seeds. Based on our preliminary investigation (data not shown), we observed that large sized fruits (unripened and ripened) possessed seeds of different maturity. Seed maturity is generally defined by its fresh weight (FW) and initial moisture content (IMC). In C. guianensis, seeds were categorized into two classes: mature (DW – 0.21 g; IMC – 38%) and immature (DW – 0.08 g; IMC – 73%) (Table 2). Germination of mature seeds on basal MS medium produced healthy seedlings in 10 days (Fig. 3e) with a germination frequency of 100%, mean shoot length of 6.26±0.22 cm and root length of 3.98±0.09 cm (Table 2). Immature seeds displayed radicle protrusion only after 14 days on basal MS medium, with a maximum germination frequency of 32.0%. Seedlings from immature seeds had crinkled cotyledonary leaves and appeared stunted with a mean shoot length of 3.08±0.13 cm (Fig. 3g-h). Higher seed germination percentage in Galax urceolata using mature dark brown seeds.36 Hundred percentage of viability in seeds from mature green fruits was observed.30 Immature seeds with greater moisture content are highly sensitive to damage from desiccation.37,38,16 Therefore, mature seeds were used for further experiments and referred to as “seeds” from herein.
Table 2: Effect of seed maturity on in vitro seed germination of C. guianensis.
| Explant | Fresh weight (g) | Dry weight (g) | Moisture content (%) | Seed germination frequency (%) | Shoot length (cm)# | Root length (cm) # |
| Mature seeds | 0.36 | 0.21 | 38 | 100 | 6.26±0.22a | 3.98±0.09a |
| Immature seeds | 0.30 | 0.08 | 73 | 32 | 3.08±0.13b | 2.44±0.13b |
#Values represent mean ±SE. Different letters indicate significant difference among the treatment means analyzed using Duncan’s multiple range test (P<0.05).
Appropriate drying methods must be employed during seed storage as the rate of drying affects the desiccation tolerance.15,39 In our study, rapid drying of seeds to a desired moisture content (DMC) of up to 20% resulted in 100% germination, while it was reduced to 40% upon slow drying (Fig. 6a). This is because, the time interval for seeds to reach the DMC of 20% was 9 h and 27 h for rapid and slow drying, respectively. The results clearly indicate that the seed viability was significantly altered depending on the duration of seed exposure to the air-dry storage environment. In concurrence with our findings40 reported that desiccation tolerance is least altered by fast drying. Moisture content (MC) and storage temperature are two other critical factors that determine the seed storage behaviour. Based on the classification,16 seeds that withstand desiccation below 5% MC fall under ‘orthodox’, 5-12% MC are ‘intermediate’ and greater than 12% MC are ‘recalcitrant’. In order to determine the true nature of C. guianensis, seeds were subjected to rapid drying up to DMC of 4% (Fig. 6b). The seed germination frequency was stable until 16% DMC and steeply declined upon further reduction in the DMC. This experiment clearly revealed the recalcitrant seed storage behaviour of C. guianensis.
Storage temperature also had a profound influence on the germination potential (Fig. 5a). Seeds of 16% DMC exposed to sub-zero temperatures for less than 24 h failed to germinate. The highest seed germination frequency (100%) was recorded from the seed lot stored at 15ºC. Significant decrease in seed germination frequency was observed above or below the optimum temperature. Intermediate seeds of tropical origin undergo chilling stress at temperatures below 10ºC while prolonged storage at higher temperature results in desiccation.37,38,41 The seed viability is generally measured biochemically by tetrazolium test (TTC).42 However, seed germination provides a true measure of seed viability. Therefore, viability of seeds stored at 15ºC was evaluated in vitro every 15 d for a maximum period of 90 d. Until 45 d of storage, the seed germination frequency was 100% which gradually decreased to about 60% after 90 d (Fig. 5b). The growth profile of plantlets established from stored seeds was similar to that grown from freshly harvested seeds (Fig. 3i). Based on TTC test,43 reported decline in seed germination of Soymida febrifuga, a deciduous medicinal tree, after 20 d of storage at 7ºC. The loss of seed viability can be attributed to the denaturation or inactivation of seed proteins and enzymes upon desiccation. Seeds of different DMC were stored at 15ºC for 2 wk and the synergistic interaction of storage temperature and seed moisture content was recorded by in vitro seed germination (Table 3). The highest shoot length 7.88±0.10 cm, root length 10.10±0.35 cm and weight of the seedlings 1.136±0.05 g was observed in freshly harvested seeds with DMC of 36%. Reduction in the DMC of seeds before storage resulted in gradual decline (to about 64% in 16% DMC) in the seedling growth.
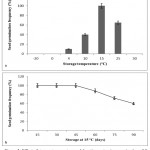 |
Figure 5: Effect of storage temperature and duration on in vitro germination of C. guianensis.
|
Germination potential of seeds (a) stored for 2 wk at different temperature regimes; (b) stored at 15°C for up to 90 d to determine the seed viability. Data recorded after 10 d of culture on MS medium containing 1.0 mg/l KIN and 0.1 mg/l IBA
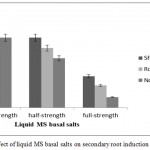 |
Figure 6: Effect of liquid MS basal salts on secondary root induction of C. guianensis.
|
Data recorded after 10 d of culture on different strength liquid MS basal salts. Different letters indicate significant difference among the treatment means analyzed using Duncan’s multiple range test (P<0.05).
Table 3: Combined effect of seed moisture content and storage temperature on in vitro seed germination of C. guianensis.
| DMC (%) | Shoot length (cm) | Root length (cm) | Weight of the seedling (g) |
| 36 | 7.88±0.10a | 10.10±0.35a | 1.136±0.05a |
| 32 | 6.70±0.32b | 8.96±0.38b | 0.968±0.02b |
| 28 | 6.20±0.27b | 8.44±0.33b | 0.857±0.01c |
| 24 | 5.44±0.16c | 6.10±0.53d | 0.832±0.01c |
| 20 | 5.26±0.13c | 7.38±0.31c | 0.787±0.02d |
| 16 | 3.98±0.25d | 5.20±0.13e | 0.736±0.01de |
Values represent mean ±SE. Different letters indicate significant difference among the
treatment means analyzed using Duncan’s multiple range test (P<0.05).
Conclusion
Conservation of medicinal plants is the need of the hour for mankind to maintain a healthy living. Most of the medicinally valuable tree species of the tropics are threatened due to the rapid destruction of their habitats. Natural propagation of tropical trees by seeds is quite difficult as they are recalcitrant, poorly developed and highly sensitive to environmental cues. In vitro embryo germination thus offers an alternative, sustainable approach to conserving the plant diversity for future generations. In the present study, we developed a simple and efficient protocol for propagation of Couroupita guianensis by germination of embryo in vitro. Healthy seedlings with 100% percent survivability under field conditions were raised by germinating mature seeds in MS medium containing PGRs within 30 days of culture. From the experiments on seed storage behaviour, it was evident that freshly harvested mature seeds of C. guianensis retained the seed germination potential even upon storage at 15ºC for up to 45 d. Exposure of seeds to air-dry storage environment significantly affected the physiological growth. These findings proved the recalcitrant seed behaviour of C. guianensis, a common phenomenon observed amongst the majority of the tropical tree species. The protocol developed herein for seed germination would thereby enable re-introduction of C. guianensis into its natural habitats.
Authors contribution statement
PSA performed the laboratory experiments, collected the data and prepared the manuscript. AV was involved in designing of experiments, data analysis and manuscript writing. RR supervised the work and provided critical comments to the manuscript.
Acknowledgments
The authors are thankful to the University Grants Commission (UGC), New Delhi [F. 39-259/2010 (SR) dated 27 December 2010] for the financial support and the management of Loyola College, Chennai, for providing the laboratory and infrastructure facilities.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be considered as a potential conflict of interest.
References
- Gousia S. K., Kumar K. A., Kumar T. V., Latha J. N. L. Biological Activities and Medicinal Properties of Couroupita guianensis. Int J Pharm Pharm Sci. 2013;3(4):140-143.
- Ramalakshmi C., Ranjitsingh A. J. A., Kalirajan K., Kalirajan A., Athinarayanan G., Mariselvam R. A Preliminary screening of the Medicinal Plant Couroupita guianensis for its Antimicrobial Potential against Clinical and Fish-borne pathogens. Elixir Appl. Bio. 2013;57:14055-14057.
- Tsou C. H., Mori S. A. Seed coat anatomy and its relationship to seed dispersal in subfamily Lecythidoideae of the Lecythidaceae (The Brazil Nut Family). Bot Bull Acad Sin. 2002;43:37–56.
- Sanz-Biset J., Campos-de-la-Cruz J., Epiquién-Rivera M. A., Canigueral S. A first survey on the medicinal plants of the Chazuta valley (Peruvian Amazon). J Ethnopharmacol. 2009;122(2):333–362.
CrossRef - Lans C., Harper T., George K., Bridgewater E. Medicinal and ethnoveterinary remedies of hunters in Trinidad. BMC Complement Altern Med. 2001; 1:10.
CrossRef - Elumalai A., Naresh V., Eswaraiah M. C., Narendar P., Raj Kumar. Evaluation of antiulcer activity of Couroupita guianensis Aubl leaves. Asian J Pharm Tech 2012;2(2):64–66.
- Gupta V. H., Wankhede S. S., Gunjal M. A., Juvekar A. R. Anxiolytic effect of Couroupita guianensis Flower Extracts in Mice. Int J Pharm Bio Sci. 2012;4(2):420–426.
- Pinheiro M. M., Fernandes S. B., Fingolo C. E., Boylan F., Fernandes P. D. Anti-inflammatory activity of ethanol extract and fractions from Couroupita guianensis Aublet leaves. J Ethnopharmacol. 2013;146(1):324–330.
CrossRef - Swapnalatha S., Rajeswari V. D. Antidiabetic activity of Couroupita guianensis. Int J Pharm Biol Sci. 2014;9(3):41–
- Mori S. A., Prance G. T. Lecythidaceae – Part II. The zygomorphic-flowered New World genera (Couroupita, Corythophora, Bertholletia, Couratari, Eschweilera & Lecythis). Fl. Neotrop. Monogr. 1990;21:1–
- Afele J. C., Langhe D. E. Increasing in vitro germination of Musa balbisiana Plant Cell Tissue Organ Cult. 1991;27(1):33–36.
CrossRef - Raghavan V. One hundred years of zygotic embryo culture investigations. In Vitro Cell Dev Biol-Plant. 2003;39(5):437–
CrossRef - Rambabu M., Upender M., Ujjwala D., Ugandhar T., Praveen M., Ramaswamy N. In vitro zygotic embryo culture of an endangered forest tree Givotia rottleriformis and factors affecting its germination and seedling growth. In Vitro Cell Dev Biol-Plant. 2006;42(5):418–
CrossRef - Fennar M. Seeds: the ecology of regeneration in plant communities. 2nd edn. CAB International, Oxford. 2002; 89(3):355-410.
- Roberts E. H., King M. W., Ellis R. H. Recalcitrant seeds: their recognition and storage. In: Holden JHW, Williams JT (eds.). Crop Genetic Resources: Conservation and Evaluation. George Allen and Unwin, London. 1984;38–52.
- Hong T. D., Linington S., Ellis R. H. Seed Storage Behaviour: a Compendium. Handbooks for Genebanks: No. 4. International Plant Genetic Resources Institute, Rome. 1996;1-115.
- Finch-Savage W. E., Blake P. S. Indeterminate development in desiccation-sensitive seeds of Quercus robur Seed science Research. 1994;4(2):127-133.
CrossRef - Berjak P., Pammentor N. M. Indeterminate development in desiccation-sensitive seeds of Quercus robur Seed science Research. 1994;4(2):263-264.
- Murashige T., Skoog F. A. A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant. 1962;15(3):473–497.
CrossRef - Bewley J. D., Seeds M. B. Physiology of development and germination. Plenum Press, 2 nd edn. New York. 1994;445.
- Bhojwani S. S., Razdan M. K. Plant Tissue Culture Theory and Practice. Elsevier, 1st Amsterdam. 1996;766.
- Taiz L., Zeiger E. (ed). Plant physiology. 3rd Sinauer Associates, Sunderland. 2006;690.
- Shukla S. P., Sharma A. In vitro seed germination, proliferation, and ISSR marker-based clonal fidelity analysis of Shorea tumbuggaia : an endangered and high trade medicinal tree of Eastern Ghats. In Vitro Cell.Dev. Biol.—Plant. 2017;53(3):200-208.
CrossRef - Salvi N. D., Singh H., Tivarekar S., Eapen S. Plant regeneration from different explants of neem. Plant Cell Tissue Org Cult. 2001;65(2):159–162.
CrossRef - Cerdas L. V., Guzman L. A. Organogénesis in vitro en Dalbergia retusa (Papilonaceae). Rev Biol Trop. 2004; 52(1):41–
- Fogaca C. M., Fett-Neto A. G. Role of auxin and its modulators in the adventitious rooting of Eucalyptus species differing in recalcitrance. Plant Growth Regul. 2005;45(1):1–10.
CrossRef - Chen Y., Cao F. L., Li S. X., Dao D. W., Xu C. P. Establishment of highly efficient regeneration system with different explants of Sapium sebiserum in vitro. Acta Bot Boreali-Occidential Sinica. 2010;30(12):2542–
- Malik S. K., Kalia R. K., Chaudhury R. In vitro regeneration of Garcinia indica using leaf explants. Ind J Plant Physiol. 2010;15(3):262–266.
- Mori Y., Miyahara F., Tsutsumi Y., Kondo R. Effects of combinational treatment with ethephon and indole-3-butyric acid on adventitious rooting of Pinus thunbergii Plant Growth Regul. 2011;63(3):271–278.
CrossRef - Ghorpade R. P., Chopra A., Nikam T. D. In vitro zygotic embryo germination and propagation of an endangered Boswellia serrata , a source of boswellic acid. Physiol Mol Biol Plants. 2010;16(2):159–165.
CrossRef - Chang S. H., Yang J. C. Enhancement of plant formation from embryo cultures of Taxus mairei using suitable culture media and PVP. Bot Bull Acad. 1996;37:35–
- Grout B. W. W., Aston M. J. Transplanting cauliflower plants regenerated from meristem culture. I. Water loss and water transfer related to changes in leaf wax and to xylem regeneration. Hort Res. 1977;17:1–
- Ziv M. In vitro hardening and acclimatization of tissue culture plants. In: Withers LA, Anderson PG (eds.). Plant tissue culture and agricultural applications. Butterwoths, London, UK. 1986;187–196.
CrossRef - McClelland M. T., Smith M. A. L., Carothers Z. B. The effects of in vitro and ex vitro root initiation on subsequent microcutting root quality in three woody plants. Plant Cell Tissue Organ Cult. 1990;23(2):115–123.
CrossRef - Shekhawat M. S., Manokari M. In vitro propagation, micromorphological studies and ex vitro rooting of cannon ball tree (Couroupita guianensis): a multipurpose threatened species. Physiol Mol Biol Plants. 2016;21(1):131-142.
CrossRef - Yang G., Shen X., Jackson R., Lu Z. C. Factors affecting in vitro seed germination and shoot proliferation of galax [Galax urceolata (Poir.) Brummitt]. Aust J Crop Sci. 2013;7(11):1766–1771.
- Ellis R. H., Hong T. D., Roberts E. H. An intermediate category of seed storage behaviour? I. Coffee. J Exp Bot. 1990;41(230):1167–1174.
CrossRef - Ellis R. H., Hong T. D., Roberts E. H. Seed moisture content, storage, viability and vigour. Seed Sci Res. 1991;1:275–279.
CrossRef - Pritchard H. W. Water potential and embryonic axis viability in recalcitrant seeds of Quercus rubra. Ann Bot. 1991;67(1):43–
CrossRef - Farrant J. M., Berjak P., Pammenter N. W. The effect of drying rate on viability retention of recalcitrant propagules of Avicennia marina. S Afr J Bot. 1985;51(6):432
CrossRef - Ellis R. H., Hong T. D., Roberts E. H., Soetisna U. Seed storage behaviour in Elaeis guineensis. Seed Sci Res. 1991;1(2):99–104.
CrossRef - Wen B. Storage of recalcitrant seeds a case study of the Chinese fan palm, Livistona chinensis. Seed Sci Technol. 2009;37(1):167–179.
CrossRef - Chiruvella K. K., Mohammed A., Ghanta R. G. Factors influencing the seed germination of Soymida febrifuga (Roxb.) A. Juss. (Meliaceae). Trakia J Sci. 2010;12(2):121–131.

This work is licensed under a Creative Commons Attribution 4.0 International License.





