Manuscript accepted on : 11-September-2018
Published online on: 24-09-2018
Plagiarism Check: Yes
The Morphological Effects of Methandienone on Sperm Head in Male Mice
Marwah Y. Falih, Abbas A. Mohammed and Ghassan M. Sulaiman
Biotechnology Division, Applied Science Department, University of Technology, Baghdad, Iraq.
Corresponding Author E-mail: xxmarwa87xx@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2671
ABSTRACT: The objective of the present study is to detect the effects of methandienone on sperm head morphology of male (Mus musculus) mice. For this purpose 55 adult male mice were used and divided into five groups: both of the control -ve and control +ve (CFA) have consisted of 5 mice, and the other three treated groups consisted of 15 mice injected orally with three doses (low, medium and high) (0.125, 0.25, 0.5) mgkg body weight methandienone, with periods (7, 21, 35) days. At the end of the treatment periods morphological abnormalities of sperm were examed. The results showed the presence of abnormal change in the form of the sperms head morphology, the effect of methandienone was dose and time dependents. The significant abnormal shapes of sperms head were observed; they were swelling head, head acrosome loss, apical hook, hammerhead, acrosome defective and other abnormalities. From these results, we can conclude Methadinone has the potential to increase the rate of deformity of head sperm morphology when increasing dose and duration of the drug, indicating that may be a genetic damage happened affects the stages of sperm formation and this needs further studies and in spite of methandienone medical uses and advantages, dose and period of treatment must be determined by a doctor.
KEYWORDS: Cyclophosphamide; Methandienone; Mice; Sperm Head Abnormalities
Download this article as:| Copy the following to cite this article: Falih M. Y, Mohammed A. A, Sulaiman G. M. The Morphological Effects of Methandienone on Sperm Head in Male Mice. Biosci Biotech Res Asia 2018;15(3). |
| Copy the following to cite this URL: Falih M. Y, Mohammed A. A, Sulaiman G. M. The Morphological Effects of Methandienone on Sperm Head in Male Mice. Biosci Biotech Res Asia 2018;15(3). Available from: https://www.biotech-asia.org/?p=31006 |
Introduction
Methandienone is an anabolic androgenic steroids drug, has been synthesized and derived from testosterone hormone (Figure 1), it works to help increase the building of proteins by increasing protein metabolism, methandienone is used for medical purposes in the treatment of many diseases such as anemia, which considered an Anti-anemia drugs because it stimulates the building of red blood cells and has been proven its effective role in the treatment of wounds, As adopted in the treatment of corneal ulcer,1,2 aplastic anemia, delayed puberty, climacterium virile, Bone fractures and Burns and used for non-medical purposes by athletes and young men in gymnasiums for increasing physical strength and muscle volume as a result of misuse by athletes and young men for its apparent effect on physical strength was banned by the World Olympic Committee.3 Taking this drug for long periods leads to hypogonadotrophic hypogonadism and may develop for the need of medical intervention.4
Methandienone had an effect on the number of sperms in rats, as it was observed in a study increasing in the number of dead sperms of rats treated with this drug and decrease in the number of live sperms,1 Some doctors are still unaware of the fact that the use of exogenous androgens inhibits the hypothalamic-pituitary-gonadal axis (HPG) and this results infertility by reducing intra-testicular testosterone.5 When Anabolic Androgenic Steroids are taken with adequate dosages for increasing muscle building they lead to increase High-Density-Lipoprotein (HDL) and decrease in Light-Density-Lipoprotein (LDL) which may lead to suppressing the production of endogenous testosterone. In medical studies, the drug was administered with a dose of 15 mg per day in males resistance training for eight weeks caused a decrease in the level of plasma testosterone to 69% without using of testosterone stimulating drugs.4 There are some common names of Methandienone and trade names such as Dianabol®, Reformist-b®, Methandrostenolone,1 Metandienone,3 Danabol, Naposim.4
![Figure 1: The chemical structure of Methandienone [4].](https://www.biotech-asia.org/wp-content/uploads/2018/09/Vol_15_no3_Mor_Mar_fig1-150x150.jpg) |
Figure 1: The chemical structure of Methandienone [4].
|
Material and Methods
Chemicals
Methadenione was supplied from LA Pharma S.r.l. (Thailand); Cyclophosphamide was purchased from Zydus (Germany). All other chemicals and reagents were used at analytical grade level.
Dose Selection
Methandienone was obtained from LA Pharma S.r.l. (Thailand). Although the human exposure dose-levels are variable 175 mg weekly5 or more, a dose of 0.125 mg/kg and double of it, 0.25 mg/kg, and double of it 0.5 mg/kg, have been used in the experiment, which also fall within human exposure dose-level, were used
Laboratory Animals
Fifty-five adult male albino mice (Mus musculus) (25±2) gm and (8-12 weeks) age were purchased from the National Center for Drug Control and Research, Baghdad, Iraq. The animals were procedured, maintained, and used in accordance with ‘Guide for the Care and Use of Laboratory Animals, and approved by the University of Technology (Baghdad, Iraq), Animal Ethical Committee. Under controlled condition (25±1) °C and given free access to food and tap water ad libitum.
Experimental Design
Animals were divided into five groups distributed as follows:
The first group (control -ve) consisted of (5) mice were injected with 0.1ml of distilled water.
The second group (control +ve) consisted of (5) mice were injected with 20 mg/kg of Cyclophosphamide, under the intraperitoneal (I.P) membrane before 24 hr. from the dissection. The third group which consisted of (15) mice were orally injected with 0.125 mg/kg body weight from the methandienone the group was subdivided into three subgroups: (G3A, G3B and G3C) each one of the subgroups consisted of 5 mice and were sacrificed after (7, 21 and 35) days of treatment respectively. The fourth group which consisted of (15) mice were orally injected with 0.25 mg/kg body weight from the methandienone the group was subdivided into three subgroups: (G4A, G4B, and G4C) each one of the subgroups consisted of 5 mice and were sacrificed after (7, 21, and 35) days of treatment. The fifth group which consisted of (15) mice were orally injected with 0.5 mg/kg body weight from the methandienone the group was subdivided into three subgroups: (G5A, G5B, and G5C) each one of the subgroups consisted of 5 mice and were sacrificed after (7, 21 and 35) days of treatment respectively.
Detection of Sperm Abnormalities
The method of Wyrobek and Bruce6 was used to obtain the sperm with some minor modifications to it. The epididymis was carefully separated from the testis and placed in a Petri dish containing 5 ml of neutral saline solution (0.58%), cut and homogenized using a Needle and sharp forceps to very small parts Then the suspension was placed in a clean test tube and left for 60 minutes, after that added fixative solution and left for half an hour.
An adequate amount of sperm suspension was placed on a slide and air dried and then stained with hematoxylin for 15 minutes, then washed with tap water and air dried, then stained with Eosin for 10 minutes, washed with alcohol and dried. The slides were examined by a light microscope (Olympus-Japan) with a magnification of 40X, to assess Morphology; the sperms were classified into 6 main categories: swelling head, head acrosome loss, apical hook, hammer head, acrosome defective and other abnormalities.
To count the sperms, we used a Hemocytometer; ten replicate counts were used for each subject to detect sperms head abnormalities.
Statistical Analysis
Statistical analysis of the obtained data was shown as Mean± SE for all groups and subgroups. The differences were estimated between the control- group and the mean values of each group by extract the value of the t-test, P<0.05 was statistically significant.
Results and Discussion
Three doses of methandienone (0.125, 0.25, and 0.5) mg/kg body weight for (Mus musculus) mice and for periods (7, 21, and 35) days of treatment were performed to evaluate the ability of the drug to induce distortions in sperms head, some of these distortions include swelling head, head acrosome loss, apical hook, hammer head, acrosome defective and other abnormalities (Ribbon shape, pin shape, irregular head shape and other forms) as shown in figure 2.
The test of distortions in the head of the sperms is being one of the important tests to detect the susceptibility of chemical and physical factors to stimulate carcinogenes or mutgens in sexual cells as it was found to be the most sensitive test 100% to the mutant materials, and it has the advantage of being low-cost and rapid tests, no need many materials or chemical processes. Also, this assay will be examined within five weeks after the treatment.7 The Tables 1-3 show the means and standard errors of these changes after (7, 21 and 35) days of treatment in comparison with the control groups.
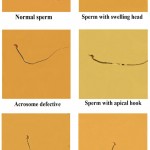 |
Figure 2: The normal sperm and different forms of abnormalities in the sperm head of mice treated with methandienone (40X).
|
During the first week of treatment, CFA administration caused statistically significant (p < 0.01) increases abnormality of sperm head morphology in comparison with the (negative control) group Table 1. and as known CFA is a drug used as an anti-cancer, which breaks down the structure of DNA and affects most of the metabolic processes within the cell. Thus this drug has a potential for cellular toxicity (cytotoxic).
Table 1: Effect of methandinone in mice sperms after one week of treatment.
| Groups | Swelling head | acrosome loss Head | Apical hook | Hammer head | Acrosome defective | Other abnormalities | Abnormalities summation | p-value by t-test |
| Mean± SE | Mean± SE | Mean± SE | Mean± SE | Mean± SE | Mean± SE | Mean± SE | ||
| Control -ve | 2.50±0.50 | 2.30±0.55 | 1.60±0.45 | 0.10±0.10 | 0.20±0.10 | 0.59±0.21 | 7.29±0.32 | P< 0.01 |
| Control +ve (CFA) | 10.50±0.60 | 5.50±1.19 | 6.80±1.16 | 0.30±1.15 | 0.80±1.15 | 2.60±0.65 | 26.50±0.98 | |
| 0.125 mg/kg | ±0.651.44 | ±0.691.60 | 2.00±0.40 | ±0.370.83 | ±0.501.30 | ±0.381.33 | ±0.508.50 | P<0.05 |
| 0.25 mg/kg | ±0.694.33 | ±1.552.67 | ±0.632.55 | ±0.401.00 | ±1.852.44 | 1.01±1.70 | ±1.1414.00 | P<0.01 |
| 0.5 mg/kg | 4.90±7.33 | ±1.102.60 | ±1.511.60 | ±1.352.33 | ±1.165.44 | ±0.811.50 | ±1.8120.80 | P<0.01 |
The statistical analysis confirmed that t-test showed a significant difference in P<0.05 when comparing the total deformities of the treated mice with 0.125 mg/kg compared to the negative standard sample, while the two doses increased 0.25 and 0.5 mg/kg in the rate of deformities of the sperm during the first week of treatment compared to negative control was the difference level (P <0.01).
Table 2. shows the average of distortions in the head of sperms after three weeks of treatment, which appear when comparing between the control negative and positive t-test there is a fundamental difference and the probability is (P <0.01 ), while the samples 0.125 and 0.25 mg/kg showed a significant difference and the probability of (P <0.01) when compared with the negative standard sample, while the difference in the high dose 0.5 mg/kg was (P <0.05).
Table 3. represents the results after five weeks of treatment, the level of self-mutations Spontaneous in the mice control negative (7.29) The level of malformations in CFA-treated mice was 26.50 and the difference between the two samples was (P <0.01), while the abnormalities values in the methandienone-treated mice were increased by (25.73, 30.75, 53.34) for the three doses (0.125, 0.25, and 0.5) mg/kg body weight respectively, all of which differed substantially (P <0.01) when compared with the negative sample. The results showed that the effect of the drug in the rate of occurrence of abnormalities in the head of the sperm after (35, 21) days for most of the distortions, which means that the drug may affects the stage of cells.
Table 2: Effect of methandienone in mice sperms after three weeks of treatment.
| Groups | Swelling head | acrosome loss Head | Apical hook | Hammer head | Acrosome defective | Other abnormalities | Abnormalities summation | p-value by t-test |
| Mean± SE | Mean± SE | Mean± SE | Mean± SE | Mean± SE | Mean± SE | Mean± SE | ||
| Control -ve | 2.50±0.50 | 2.30±0.55 | 1.60±0.45 | 0.10±0.10 | 0.20±0.10 | 0.59±0.21 | 7.29±0.32 | P< 0.01 |
| Control +ve (CFA) | 10.50±0.60 | 5.50±1.19 | 6.80±1.16 | 0.30±1.15 | 0.80±1.15 | 2.60±0.65 | 26.50±0.98 | |
| 0.125 mg/kg | 6.40±0.40 | 2.00±0.54 | 1.20±0.25 | 1.60±0.40 | 4.80±1.28 | 1.00±0.32 | 17.00±0.53 | P< 0.01 |
| 0.25 mg/kg | 6.80±1.46 | 3.00±0.71 | 1.80±0.40 | 2.20±0.32 | 5.20±0.45 | 1.40±0.40 | 20.40±0.62 | P< 0.01 |
| 0.5 mg/kg | 8.40±0.25 | 3.20±1.34 | 1.60±0.33 | 2.00±1.00 | 5.80±0.88 | 1.60±0.66 | 22.60±0.74 | P< 0.05 |
Table 3: Effect of methandienone in mice sperms after five weeks of treatment.
| Groups | Swelling head | acrosome loss Head | Apical hook | Hammer head | Acrosome defective | Other abnormalities | Abnormalities summation | p-value by t-test |
| Mean± SE | Mean± SE | Mean± SE | Mean± SE | Mean± SE | Mean± SE | Mean± SE | ||
| Control – ve | 2.50±0.50 | 2.30±0.55 | 1.60±0.45 | 0.10±0.10 | 0.20±0.10 | 0.59±0.21 | 7.29±0.32 | P< 0.01 |
| Control +ve (CFA) | 10.50±0.60 | 5.50±1.19 | 6.80±1.16 | 0.30±1.15 | 0.80±1.15 | 2.60±0.65 | 26.50±0.98 | |
| 0.125 mg/kg | 9.30±0.68 | 5.20±0.61 | 1.83±0.31 | 1.50±0.34 | 6.30±0.63 | 1.60±0.34 | 25.73±0.49 | P<0.01 |
| 0.25 mg/kg | 10.50±1.71 | 5.33±0.67 | 2.10±0.28 | 2.50±0.76 | 8.02±0.58 | 2.30±0.56 | 30.75±0.76 | P<0.01 |
| 0.5 mg/kg | 13.40±0.82 | 5.00±0.54 | 2.50±0.43 | 3.30±1.03 | 8.00±0.64 | 2.33±0.67 | 34.53±0.69 | P<0.01 |
The stage containing the primary spermatocytes and secondary spermatocytes that these cells were more sensitive to the drug and this is consistent with the studies of many chemicals and some medicines, an increase in the rate of abnormalities of the sperm head of the rats treated with cyclophosphamide drug,8 as we can see in this study the concentration of 0.125 mg/kg body weight for laboratory mice had the lowest percentage of head abnormalities. Taking too little dose of methandienone will not have a side effect but taking large amounts and for long periods may cause serious side effects. The formation and maturity of sperm are regulated and controlled by a number of genes.9 Thus, these abnormalities of the head of the sperm can result from the chromosomal aberration that appears during the process of filling the genetic material in the head of the sperm or the result of the mutation Point Mutation in Testicular DNA due to the exposure of the organism to chemical agents10 and damage can occur in the sperm cell due to physical factors, The incidence of malformations in the head of the sperm of radio-exposed mice11 has been increased. Such abnormal adverse effects are known to be caused by the effects of lysosomes and diseases called lysosomal storage diseases affect the plasma membrane of sperm cells.12
These abnormalities in sperm coincide with Infertility in men,13 due to the effect on genetically modified sperm. In many studies it has been observed that the high rate of deformities affecting the head of the sperm affect fertility, as it was observed to be associated with changes and damage in the genetic material DNA in the stages of pre-meiotic phase of the process of sperm formation.9,10,11,12 These abnormalities may affect on fertilization and pregnancy, because the abnormal shape of the sperm is under genetic control and therefore the effect may be the increase in the deposition of fat that affects the diameter of the conveyor seminiferous tubules or too clogged and inability to move and may cause the incidence of infertility.
Conclusion
Methadinone has the potential to increase the rate of deformity of head sperm morphology when increasing dose and duration of the drug, indicating that may be a genetic damage happened affects the stages of sperm formation and this needs further studies.
Acknowledgment
The authors are thankful to the management of biotechnology division, University of Technology for providing the basic facilities to perform this research work.
Conflict of interest statement
Authors would like to declare that there is no conflict of interest.
References
- Jassim A. M., ALZamely H. A. N., Hamad A. G. Study of the testicular damage induced by dianabol and its effect on morphological and histological changes in albino male rats. IOSR-JAVS. 2015;8(1):24-32.
- Pope H., Wood R., Rogol A., Nyberg F., Bowers L., Bhasin S. Adverse health concequences of performance. Enhancing drugs: an Endocrine Society scientific statement. Endor. Res. 2013;35(3):341-375.
CrossRef - Parr M. K., Geyer H., Hoffmann B., Köhler K., Mareck U., Schänzer W. High amounts of 17‐methylated anabolic‐androgenic steroids in effervescent tablets on the dietary supplement market. Biomedical chromatography. 2007;21(2):164-168.
CrossRef - Llewellyn’s W. (6th) Anabolics 2007, 6th edn. Body of Science.USA. 2007;176-180.
- Rahnema C. D., Lipshultz L. I., Crosnoe L. E., Kovac J. R., Kim E. D. Anabolic steroid–induced hypogonadism: diagnosis and treatment. Fertility and Sterility. 2014; 101(5):1271-1279.
CrossRef - Rashid K., Sil P. C. Curcumin ameliorates testicular damage in diabetic rats by suppressing cellular stress-mediated mitochondria and end oplasmic reticulum-dependent apoptotic death. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2015;1852(1):70-82.
CrossRef - jameel Al-Shaikhli R., Al-Janabi A. A. The cytogenetic effect of Euphorbia tirucalli stems methanolic extract on sperm head morphology in male albino mice. HOAJ Biology. 2017;6(1):1-6.
CrossRef - Selvakumar E., Prahalathan C., Sudharsan P. T., Varalakshmi P. Chemo protective effect of lipoic acid against cyclophosphamide-induced changes in the rat sperm. Toxicology. 2006;217(1):71-78.
CrossRef - Otubanjo O. A., Mosuro A. A. An in vivo evaluation of induction of abnormal sperm morphology by some anthelmintic drugs in mice. Mutation Research Genetic Toxicology and Environmental Mutagenesis. 2001;497(1):131-138.
CrossRef - Gotoh H. Inherited sperm head abnormalities in the B10. M mouse strain. Reproduction. Fertility and Development. 2010;22(7):1066-1073.
CrossRef - Otitoloju A. A., Obe I. A., Adewale O. A., Otubanjo O. A., Osunkalu V. O. Preliminary study on the induction of sperm head abnormalities in mice, Mus musculus exposed to radiofrequency radiations from global system for mobile communication base stations. Bulletin of environmental contamination and toxicology. 2010;84(1):51-54.
CrossRef - Yavari A. Abuse of anabolic androgenic steroids. Journal of stress physiology & biochemistry. 2009;5(3):22-32.
- Chenoweth P.J. Genetic sperm defects. Theriogenology. 2005;64(3):457-468.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





