Manuscript accepted on : 16-April-2018
Published online on: 27-09-2018
Plagiarism Check: Yes
Sonu Ambwani1 , Tanuj Kumar Ambwani2 and Ramswaroop Singh Chauhan3
, Tanuj Kumar Ambwani2 and Ramswaroop Singh Chauhan3
1Department of Molecular Biology and Genetic Engineering, College of Basic Sciences and Humanities, GBPUA and T, Pantnagar, Uttarakhand, India.
2Department of Veterinary Physiology and Biochemistry, College of Veterinary and Animal Sciences, GBPUA and T, Pantnagar, Uttarakhand, India.
3Department of Veterinary Pathology, College of Veterinary and Animal Sciences, GBPUA and T, Pantnagar, Uttarakhand, India.
Corresponding Author E-mail: ambwani_sonu@rediffmail.com
DOI : http://dx.doi.org/10.13005/bbra/2679
ABSTRACT: Cow urine has many beneficial properties particularly in the areas of agriculture and therapeutics. It has also been observed during the scientific research that the urine of Indian cows is highly effective against various ailments. Cow urine or ‘gau mutra’ has a unique place in Ayurveda and is suggested for improving general health. Cypermethrin is a widely used composite pyrethroid. It is a broad spectrum, non-cumulative insecticide and a fast-acting neurotoxin. It is reported to exhibit deleterious health impacts on human and/ animal health. Present paper reports ameliorating effects of cow urine distillate (CU) against cypermethrin induced immunotoxicity and oxidative stress in chicken lymphocytes culture employing lymphocyte proliferation assay and nitric oxide (NO) estimation. Cypermethrin treated cells displayed immunotoxic effects as observed by decrease in B and T cells proliferation. In case of combination treatments of cypermethrin and CU, there was increase in B and T cells proliferation as compared to only pesticide treated cells. Nitric oxide estimation revealed enhanced oxidative stress in cypermethrin treated cells in comparison to combination treated groups.
KEYWORDS: Chicken Lymphocytes; Cow Urine; Cypermethrin; Immunotoxicity; Oxidative Stress
Download this article as:| Copy the following to cite this article: Ambwani S, Ambwani T. K, Chauhan R. S. Ameliorating Effects of Badri Cow Urine on Cypermethrin Induced Immunotoxicity and Oxidative Stress in Chicken Lymphocytes Culture System. Biosci Biotech Res Asia 2018;15(3). |
| Copy the following to cite this URL: Ambwani S, Ambwani T. K, Chauhan R. S. Ameliorating Effects of Badri Cow Urine on Cypermethrin Induced Immunotoxicity and Oxidative Stress in Chicken Lymphocytes Culture System. Biosci Biotech Res Asia 2018;15(3). Available from: https://www.biotech-asia.org/?p=31240 |
Introduction
Pesticides are the man made chemicals which are being used to enhance agricultural productivity to ensure food security. In India, large quantities of pesticides are used annually to control pests and plant diseases. However, there are many reports available citing deleterious health effects of pesticides in man and animals including immunomodulatory effects. In recent years, interest has been generated among scientific community of the world to develop or scientifically validate the Indigenous Technical Knowledge (ITK) as an alternate therapeutic or preventive approach. The ancient Indian systems of medicine, Ayurveda, avow many beneficial implication of Panchgavya in treatment of a range of ailments apart from its use in agriculture, organic farming as good quality natural manure, biopesticides, bio-fertilizer, pest repellants and as alternate energy resources (biogas, fuel and electricity), etc. This preventive approach is also known as ‘cowpathy’. Cow urine was found to enhance humoral and cell mediated immune responses by inducing B and T cells blastogenesis and increased the level of IgG in mice. It is further reported that cow urine increased levels of IL-1 and IL-2 in mice and rat as found through in vivo studies (Chauhan et al., 2001, 2004).
Cypermethrin (IUPAC: (RS)-ý-cyano-3-phenoxybenzyl (1RS)-cis, trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate; Molecular formula: C22H19Cl2NO3; Molecular weight: 416.32) is a composite pyrethroid and a fast-acting neurotoxin. It is active against a wide range of insect pests. Elimination of cypermethrin was rapid in most animals, in most tissues the half-life was approximately one day. However, it accumulates in environment and food chain leading to deleterious effects in animals and man. The toxic effects and NOEL dose of cypermethrin is well documented (WHO/FAO, data sheet on pesticides No. 58; Gahukar, 1999). The pesticide dose used was NOEL/103 while 100 times diluted CU was used in the present study carried out employing chicken lymphocytes culture system. In the study efforts were made to evaluate ameliorating potential of cow urine against cypermethrin induced immunotoxicity and oxidative stress.
Materials and Methods
Chicken lymphocytes
Chicken spleen was collected from healthy birds from local slaughter house, and lymphocytes were isolated under laminar air flow as per the standard procedure (Janossy and Greaves 1971). Lymphocytes were separated through density gradient centrifugation (Histopaque 1077, Sigma) as per the method described by Rose and Friedman (1976).
Cell viability Assay
Percentage cell viability was determined by 0.1 per cent trypan blue dye exclusion test using haemocytometer (Boyse et al., 1964) and final cell count was adjusted to 107 cells/ ml in RPMI-1640 medium and made into one ml aliquots in eppendorf tubes and cells were pelleted by centrifugation at 1400 rpm for 10 min.
Pesticide Treatment
Commercial preparation of cypermethrin was purchased from local market and it’s thousand times diluted NOEL (5.0 mg/ kg body weight) dose in RPMI- 1640 medium (Hi – Media, India) was used for the in vitro exposure of avian lymphocytes for 2 hours (hr) at 37oC. After incubation, cells were washed twice and finally suspended in 1 ml of RPMI-1640 medium supplemented with 10% FCS (Sigma, USA).
Cow Urine Treatment
Cow urine was collected from the Badri cow inhabited in hilly area of Uttarakhand (now named as Badri cow). Cow urine was collected and its distillate was prepared by 50 per cent distillation and stored in sterile air tight containers till further use. The cow urine distillate (CU) was diluted hundred times in RPMI-1640 medium and used in the experiments. Two combination treatments of pesticide (NOEL/103 dilution) and 1:100 times diluted CU were used. In first combination treatment group, cell pellet was pre-treated with cow urine for 1 hr followed by pesticide for 1 hr at 37°C. In second combination treatment group, cells were co-treated with pesticide and cow urine for 2 hr at 37°C.
Lymphocyte Proliferation Assay (LPA)
LPA or B & T cell blastogenesis assay was carried out as per the method described by Rai-el-Balhaa et al. (1985) and modified by Chauhan (1998). Concanavalin-A (ConA) (Sigma, USA) was used as a T cell mitogen whereas lipopolysaccharide (LPS) (Sigma, USA) as a B cell mitogen at a concentrations of 5 µg/ml, each, in RPMI-1640 medium.
Statistical Analysis
Analysis of variance (ANOVA) and student’s t-test were used to estimate significant difference between control and treated cells. The values were expressed as mean delta Optical Density ± standard error (mean Δ OD± SE). Student t-test was employed for comparing the mean ODs (Snedecor and Cochran, 1967).
Oxidative Stress Assay
Macrophages were isolated from spleen on the basis of their adherent properties (Wigley et. al., 2001). Cypermethrin treated and control cells were seeded in 24-well culture plates. Cells were then incubated at 37oC in 5% CO2 for 4 hr to allow the adherence of macrophages. After incubation, cells were washed vigorously four times with DMEM to remove non-adherent cells. These cells were incubated at 37oC in CO2 incubator in presence of LPS (Sigma) at a concentration of 5 µg/ml. Nitric oxide (NO) production by macrophages in the medium was measured by microplate assay method (Stuehr et al., 1988). The standard curve to calculate the NO production was prepared using different dilutions of NaNO2.
Results
LPA
The in vitro exposure of avian lymphocytes to NOEL/103 dose of cypermethrin showed significant decrease in B cell blastogenesis in the presence of B cell mitogen (LPS). In case of combination treatments, CU pre-treated cells showed the maximum increase in B cell blastogenesis with mean delta O.D. 0.346 ± 0.013 followed by simultaneous CU treated cells with mean delta O.D.s of 0.239 ± 0.003. Over all, there was 56.12 per cent decrease in B cell blastogenesis in cypermethrin treated cells while 2.64 per cent increase in B cell blastogenesis in CU treated cells (Table- 1 and Fig.- 1).
Table 1: In vitro effects of cypermethrin and cow urine on B cell blastogenesis in avian lymphocytes.
| S. No. | Treatments | Mean ΔO.D. ± S.E.** | Percentage change |
| 1. | Control | 0.417 ± 0.001 | – |
| 2. | Cow Urine | 0.428 ± 0.002 | +2.64 |
| 3. | Cypermethrin | 0.183 ± 0.003 | -56.12 |
| 4. | CU→Cyp. | 0.346 ± 0.013 | -17.03 |
| 5. | CU : Cyp. | 0.239 ± 0.003 | -42.69 |
| CD at 1% = 0.027 CD at 5% = 0.019 | |||
** Significant at p< 0.01
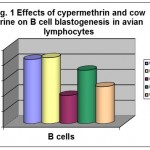 |
Figure 1: Effects of cypermethrin and cow urine on B cell blastogenesis in avian lymphocytes.
|
Cypermethrin treated avian lymphocytes showed marked decrease in T cell blastogenesis in the presence of mitogen ConA. CU treated cells showed slight increase in the blastogenesis as compared to the control. Among the two combination treatments, pre-treatment and co-treatment with CU showed almost an equal increase in T cell blastogenesis as compared to cypermethrin treated cells. There was 56.22 per cent decrease in T cell blastogenesis in cypermethrin treated cells while 17.60 percent increase in T cell blastogenesis in CU treated cells (Table- 2 and Fig.- 2).
Table 2: In vitro effects of cypermethrin and cow urine on T cell blastogenesis in avian lymphocytes.
| S. No. | Treatments | Mean ΔO.D. ± S.E.** | Percentage change |
| 1. | Control | 0.466 ± 0.018 | – |
| 2. | Cow Urine | 0.548 ± 0.015 | +17.60 |
| 3. | Cypermethrin | 0.204 ± 0.016 | -56.22 |
| 4. | CU→Cyp. | 0.243 ± 0.004 | -47.85 |
| 5. | CU : Cyp. | 0.239 ± 0.023 | -48.71 |
| CD at 1% = 0.070 CD at 5% = 0.050 | |||
** Significant at p< 0.01
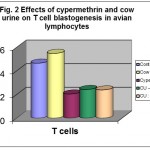 |
Figure 2: Effects of cypermethrin and cow urine on T cell blastogenesis in avian lymphocytes.
|
Oxidative Stress Assay
Oxidative stress was detected by NO estimation. As illustrated in the Table- 3 and Fig.- 3 cypermethrin treated cells exhibited more NO concentration as compared to the control. Oxidative stress was detected by nitric oxide (NO) estimation. Among the two combination treatment groups, CU pre-treated cells showed the least NO production having a mean concentration 92.38 ± 0.669µM/ml. Cow urine distillate treated cells showed minimum NO production with 44.02 ± 0.913 µM/ml as compared to the control with mean NO concentration of 74.68 ± 0.841µM/ml.
Table 3: In vitro effects of cypermethrin and cow urine on NO concentration (µM/ml) in mononuclear cells.
| S. No. | Treatments | Mean Conc. ± S.E.** | Percentage change |
| 1. | Control | 74.68 ± 0.841 | – |
| 2. | Cow Urine | 44.02 ± 0.913 | -41.06 |
| 3. | Cypermethrin | 205.09 ± 1.475 | +174.62 |
| 4. | CU→Cyp. | 92.38 ± 0.669 | +23.70 |
| 5. | CU : Cyp. | 105.23 ± 0.897 | +40.91 |
| CD at 1% = 6.283 CD at 5% = 6.484 | |||
** Significant at p< 0.01
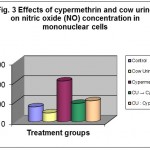 |
Figure 3: Effects of cypermethrin and cow urine on nitric oxide (NO) concentration in mononuclear cells.
|
Discussion
The acute toxicity of many pesticides is well known. However, much less is known about longer-term impacts on different systems of the human body including the nervous, endocrine, reproductive and immune systems. Pyrethroid insecticides have been used in agricultural and home formulations for more than 30 years (Casida and Quistad, 1998). Continuous exposure of pesticides even at low dose levels can exert adverse effects on immune system. The present study was planned to examine the ameliorating effects of CU against cypermethrin induced immunotoxicity and oxidative stress in chicken lymphocytes cell culture system.
In the study conducted, there was a decrease in B and T cell blastogenesis and increase in oxidative stress in cypermethrin treated cells as compared to control cells (Ambwani, 2004; Ambwani et al., 2006). This was in good agreement with several other workers. Studies have found permethrin, a synthetic pyrethroid insecticide to be toxic to the immune system. Permethrin inhibited the mitogentic response of murine splenic lymphocytes to concanavalin-A and lipopolysaccharide (Stelzer and Gordan, 1984). Topical exposure to permethrin was found to cause reduction of macrophage function and antibody production in the spleen, indicating that exposure may produce systemic immune effects (Punareewattana et al., 2001). Ambwani et al. (2010, 2012) reported immunotoxic effect due to allethrin exposure in chicken lymphocytes. Chauhan and Agrawal (1999) studied immunopathological effects of alphamethrin, a synthetic pyrethroid, in six cross bred male bovine calves. The results showed that the blastogenic activity of T and B lymphocytes was reduced by 48 and 40 per cent, respectively in comparison to the controls. The present study displayed enhanced oxidative stress through NO estimation in cypermethrin treated cells (Ambwani, 2004; Ambwani et al., 2006). There is a clearly established relationship between ROS/ free radicals and apoptosis. Since ROS/ free radical intermediates mediate many immune cell functions and apoptosis has been established in immune cell populations, it is likely these two events could arise simultaneously during certain chemical exposures. In particular, two different studies reported induced apoptosis in rat and murine thymocytes and concluded an association between the onset of apoptosis and the increase in ROS (Beaver and Waring, 1995; Bustamante et al., 1997). There is a recent report establishing relationship between oxidative stress and genotoxicty due to cypermethrin exposure in swiss albino mice (Srivastava et al., 2012).
Recent researches report that cow urine enhances the immune status of an individual through activating the macrophages and augmenting their engulfment power (Chauhan, 2013). In poultry, cow urine enhances the immunocompetence of birds and provides better protection. The cow urine has been reported to be of great therapeutic value in wide spectrum of diseases (Ray et al., 1980; Chauhan et al., 2004; Chauhan and Singhal, 2006). Randhawa (2010) reviewed bioenhancer properties of cow urine distillate. Cow urine has potent immunomodulatory effect and is capable of enhancing both cellular and humoral immune responses (Ambwani, 2004; Ambwani et al., 2006; Ganguly and Prasad, 2011). Chauhan et al. (2001) studied the immunomodulatory effect of cow urine in mice and found that cow urine enhances T & B cell blastogenesis and increases the level of IgG. It also enhances IL-1 and IL-2 level in mice (Chauhan et al., 2004). Krishnamurthi et al. (2004) reported protective effects of cow urine distillate against actinomycin-D and H202 as revealed by fluorimetric analysis of DNA unwinding (FADU). They showed that the damage could be protected with the redistilled cow’s urine distillate (1, 50 & 100 microL) in simultaneous treatment with genotoxic chemicals. Ambwani et al. (2014) reported counteracting effects of cow urine distillate against allethrin induced immunotoxicity in chicken lymphocytes. Recently, Tadavi et al. (2017) reported that subacute exposure of chlorpyrifos @ 50 ppm in feed has adverse effect on clinical and haematological observations in broiler chickens and co-administration of cow urine distillate ameliorated these changes.
Present study clearly indicated counteracting effects of CU against cypermethrin induced immunotoxicty and oxidative stress. It would be worthwhile to further study the immunopotentiating and ameliorating effect of cow urine on pesticide induced immunotoxicity and stress at molecular level, with special reference to the detailed biochemical characterization of cow urine.
Acknowledgement
Facilities provided by Director Experiment Station and Dean, College of Veterinary and Animal Sciences, GBPUA&T, Pantnagar; to carry out present study, are duly acknowledged.
References
- Ambwani S. Molecular Studies on Apoptosis in Avian Lymphocytes Induced by Pesticides. Ph. D. thesis submitted to the G.B. Pant University of Agriculture & Technology Pantnagar, U.A., India. 2004.
- Ambwani S., Ambwani T., Singh G. K and Chauhan R. S. In vitro model system for evaluation of immuno supression oxidative stress and apoptosis in avian lymphocytes due to low level dose exposure of allethrin in Proceedings of the IIIrd Uttarakhand State Science and Technology Congress- 2008, Macmillan Advanced Research Series. 2012;93-101.
- Ambwani S., Ambwani T., Singh S. P and Chauhan R. S. Evaluation of allethrin induced immunotoxicity in avian lymphocytes. Journal of Veterinary Pharmacology and Toxicology. 2010;9(1-2):68-70.
- Ambwani S., Ambwani T., Singhal L and Chauhan R. S. Immunomodulatory effects of Cow urine on dimethoate induced immunotoxicity in avian Lymphocytes. International Journal of Cow Science. 2006;2(1):45-48.
- Ambwani S., Ambwani T and Chauhan R. S. Evaluation of immuno suppressive effects and apoptosis due to cypermethrin exposure in avian lymphocytes cell culture system. Journal of Immunology and Immunopathology. 2006;8(2):126-127.
- Ambwani S., Ambwani T and Chauhan R. S. Counteracting Effects of Cow Urine on Allethrin Induced Immunotoxicity and Oxidative Stress in Chicken Lymphocytes Culture System. Journal of Immunology and Immunopathology. 2014;16(1-2):58-63.
CrossRef - Beaver J. P and Waring P. A decrease in intra cellular glutathione concentrations precedes the onset of apoptosis in murine thymocytes. Eur. J. Cell Biol. 2014;68:47-54.
- Boyse E. A., Old L. J and Chouroulinkov I. Cytotoxic test for demonstration of mouse antibody. Method in Medical Research. Eisen. 1964;10:39-47.
- Bustamante J., Tovar B. A., Montero G and Boveris A. Early redox changes during rate thy mocyte apoptosis. Arch. Biochem. Biophys. 1997;337:121-128.
CrossRef - Casida J. E and Quistad G. B. Golden age of insecticide research past present or future. Annu Rev Entomol. 1998;43:1-16.
CrossRef - Chauhan R. S. Laboratory manual of immuno pathology G. B. Pant University of Agriculture & Technology Pantnagar. 1998.
- Chauhan R. S. Indigenous Cow Urine and Immunomodulation. Journal of Immunology and Immuno pathology. 2013;15(1):19-22.
- Chauhan R. S and Agrawal D. K. Immuno pathology of alphamethrin in bovine calves. Ind. J. Anim. Sci. 1999;69: 556-559.
- Chauhan R. S and Singhal L. Harmful effects of pesticides and their control through cow pathy. International Journal of Cow Science. 2006;2(1):61-70.
- Chauhan R. S and Tripathi B. N. Veterinary immuno pathology (Theory & practice). Lucknow. IBDC. 2002;476.
- Chauhan R. S., Singh B. P and Singhal L. K. Immunomodulation with Kamdhenu ark in mice. Journal of Immunology and Immunopathology. 2001;3:74-77.
- Chauhan R. S., Singh D. D., Singhal L. K and Kumar R. Effect of cow urine on interleukin-1 and 2. Journal of Immunology and Immunopathology. 2004;6(S-1):38-39.
- Diel F., Detscher M., Schock B and Ennis M. In vitro effect the pyrethroids s-biocypermethrin on lymphocytes and basophils from atopic and nonatopic subjects. Allergy. 1998;53:1052-1059.
CrossRef - Diel F., Horr B., Borck H., Savtchenko H., Mitsche T and Diel E. Pyrethroids and piperonyl-butoxide affect human T-lymphocytes. in vitro. Toxicol. Lett. 1999;107:65-74.
CrossRef - Gahukar R. T. Agro-medical guide of synthetic pesticides. Nagpur Agri-Horticultural Publishing House. 1999;487.
- Ganguly S and Prasad A. Role of plant extracts and cow urine distillate as immuno modulators. A review. Journal of Medicinal Plants Research. 2011;5(4):649-651.
- Krishnamurthi K., Dutta D., Sivanesan S. D and Chakrabarti T. Protective effect of distillate and redistillate of cow’s urine in human polymorphonuclear leukocytes challenged with established genotoxic chemicals. Biomed Environ Sci. 2004;17(3):247-56.
- Miers L. A., Bankowski R. A and Zee Y. C. Optimizing the enzyme linked immunosorbent assay for evaluating immunity of chickens to new castle disease. Avian Diseases. 1983;27:112-115.
CrossRef - Punareewattana K., Smith B. J., Blaylock B. L., Longstrth J., Snodgrass H. L., Gogal J. R., Prater R. M and Holladay S. D. Topical permethrin exposure inhibits antibody production and macro phage function in C57/BL6 mice. Food Chem. Toxicol. 2001;39:133-139.
CrossRef - Rai-el-Balhaa G., Pellerin J. L., Bodin G and Abdullah A. Lymphocytic transformation assay of sheep peripheral blood lymphocytes a new rapid and easy to read technique. Comp. Immunol. Microbiol. Infect. Dis. 1985;8:311-318.
CrossRef - Randhawa G. K. Cow urine distillate as bioenhancer. J Ayurveda Integr Med. 2010;1(4):240–241.
CrossRef - Ray P., Gupta H and Roy M. Susruta-Samhita (A Scientific Synopsis). New Delhi Indian National Science Academy. 1980.
- Snedecor G. W and Cochran W. E. Statistical methods. 6th Ed. New Delhi, Oxford & IBH Publication Co. 1967.
- Srivastava A. K., Srivastava P. K., Al-Khedhairy A. A., Musarrat J and Shukla Y. Cypermethrin-induced genotoxicity and oxidative stress in Swiss albino mice. Mutat Res. 2012;747(1):22-8. doi: 10.1016/j.mrgentox. 2012.03.003. Epub 2012 Mar 20.
- Stelzer K. J and Gordon M. Effect of pyrethroids on lymphocyte mitogenic responsiveness. Res. Commun. Chem. Pathol. Pharmacol.66: 137-150. (Cited in Aercotes J. ed. Immunotoxicology of drugs and chemicals. 1984;315-332. New York, Oxford.
- Stuehr D. L., Morris C and Nathan C. F. Cytostasis from nitrite a product of activated macrophages. FASEB J. 1988;2. A 1452.
- Tadavi S. B., Hedau M., Ingole R. S., Hajare S. W and Wade M. R. Clinical and haematological changes induced by chlorpyrifos and amelioration by cow urine distillate in broilers. Journal of Entomology and Zoology Studies. 2017;5(6):1510-1513.

This work is licensed under a Creative Commons Attribution 4.0 International License.





