Manuscript accepted on : 29 March 2018
Published online on: --
Plagiarism Check: Yes
Pheromone Producing Dorsal Abdominal Glands in Leptocoris Augur
Section of Entomology, Department of Zoology, Aligarh Muslim University, Aligarh, India 202002.
Corresponding Author E-mail: ayesha.zoology@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2623
ABSTRACT: Morphology of the scent (pheromone) glands is the first and basic step in understanding the mechanism of working of this highly evolved and integrated system of communication in insects. Leptocoris augur contains a pair of large and distinct symmetric exocrine dorsal abdominal glands (DAGs) found in the dorsal abdominal segments. The glands are simple, voluminous, sac-like and bright orange that turn brown and downsized with age. They are invested with a large and dense network of trachea and open to the exterior via minute slit shaped openings in the form of ostioles that remain permanently open to the outside. The functional specificity of these glands can be associated with the activities ranging from aggregation to sexual behaviours like courtship and mating. The mating is not restricted to a specific season, being distributed all-round the year; however, the insect prefers low and humid temperature for mating and they can be seen congregating in huge numbers when the hot summers subside and the rainy season arrives. We didn’t observe any considerable variation in size of these glands with respect to the body size in between the instars and the adults. Further, there were no significant difference between males and females in terms of the size of these DAGs.
KEYWORDS: DAG; Exocrine Semiochemicals;
Download this article as:| Copy the following to cite this article: Qadir I, Qamar A. Pheromone Producing Dorsal Abdominal Glands in Leptocoris Augur. Biosci Biotech Res Asia 2018;15(1). |
| Copy the following to cite this URL: Qadir I, Qamar A. Pheromone Producing Dorsal Abdominal Glands in Leptocoris Augur. Biosci Biotech Res Asia 2018;15(1). Available from: https://www.biotech-asia.org/?p=29429 |
Introduction
The communication process of invertebrates, especially insects, has fascinated scientists since long times and led them to various pathbreaking discoveries regarding communication- its effectors, modes, and mediums- and this has eventually evolved into the science of semiochemicals. These chemicals are secreted to the outside through specialised glands general body surfaces and modulate the behaviour of their recipients. Dufour (1833) published the first account of scent glands in adult Heteroptera and Kunkel (1866) was the first to observe the presence of scent gland openings on the dorsal side of the abdomen in some Heteropteran nymphs. The morphology of scent glands and the chemistry of their secretions have been studied extensively in many heteropteran species (Dazzini and Pawan, 1978; Staddon,1979, 1986; Blum, 1981; Aldrich, 1988). The glands are present as serial present cutaneous invaginations ranging from one to five (Staddon 1979). The function of these glands is usually in alarm and/or defense purposes and produce repulsive and/or toxic chemicals; however, in some true bugs, scent gland composition in adults can exhibit a strong sexual dimorphism and are involved in the sexual behaviour (Aldrich et al. 1978, 1984; Hamilton et al. 1985). In other species, the concentration of its different chemical constituents determines whether they act as command signals for aggregation and alarm (Ishiwatari, 1974; Lockwood and Story, 1985, 1987; Kou et al. 1989). There has been an immense contribution of many authors that have enriched the knowledge about morphology, histology, biological functions and components of the scent glands (Remold 1962a,1962b; Carayon 1971; Aldrich et al.1972; Qadir I, et al., 2015). Recent studies regarding morphology utilize examining electron microscopy that permit more comprehensive portrayals of structures in detail (e.g., Bianchi et al. 2011, Pollo et al. 2012, Gilio-Dias et al. 2013). Thereafter, the process of scrutinizing the exocrine secretions for their associated structures and functions continue till date and.
Soapberry bug, Leptocoris augur (Fabricius) is a sap sucking bug, found primarily feeding on the plants of Sapindaceae family which include economically important trees such as Kusum tree, boxelders, maples, soapberries (or soapnuts), jacket plums, rambutans and litchis. These insects are found in Southeast Asia mainland including India. Their ancestors are believed to have been originated from Africa where they are present as an enormous diversity. L. augur both nymphs and adults studied in the present study feed principally on the seeds of Kusum tree-Schleichera oleosa, called Koshamra in Ayurveda.
In the present study, we intended to study the changes associated with DAGs mainly in terms of morphological aspects throughout the post embryonic stages.
Experimental
Adults and nymphs of L. augur were collected from the seeds and bark of its host tree S. oleosa in the campus of Aligarh Muslim University, Aligarh, India. Insects were maintained at standard rearing conditions of temperature (25±1ºC) and humidity (70±5 %) and continuously fed on the seeds of its host plant.
Light Microscopy
For light microscopic analysis, the insects were killed by freezing and then dissected under stereoscopic microscope using fine scalpels. The dorsal side was exposed and observed for morphological details.
Body vs Gland Measurements
For gland vs body measurements Nikon Sterozoom binocular (Model no. SMZ- 1000) microscope was used (at 10x). Three independent observations were made in case of each measurement.
Scanning Electron Microscopy
The insects chosen for Scanning Electron Microscopy (SEM) were killed by freezing and their abdominal parts were cut, washed with phosphate buffer, and immediately fixed in 3% glutaraldehyde (0.1M sodium phosphate buffer, Ph=7.2) for 3 hours and subsequently rinsed thrice in fresh phosphate buffer. The post fixation was carried using 1% Osmium tetra oxide (0.1M sodium phosphate buffer, Ph=7.2) done for 4 hours and then dehydrated in gradient ethanol series, dried using Critical Point Drying Method (CPD) and coated with gold particles. The observations were made on JSM650LV scanning electron microscope (JEOL- Japan).
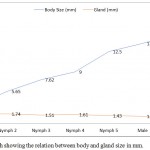 |
Figure 1: Line graph showing the relation between body and gland size in mm.
|
Results
Light Microscopy
On visualizing the dorsal side of the abdomen with unaided eye, two conspicuous orange coloured dot-like areas can be seen below the integument. It was easy for us to distinguish these areas as their colour was distinct from the rest of the tergite. They were slightly more pigmented from rest of the dorsal integument. Based on the reported literature, we could speculate these areas to be DAGs present in the middle of the 4th and 5th abdominal tergites but to validate our hypothesis, we observed these structures under a microscope. On observing these areas, two – minute openings were observed. These oval shaped slits open in the middle of these segments. And with subsequent observation, we were able to conclude that these were the openings through which DAGs, located just underneath, open to the outside. Further, as the integument was carefully removed, taking into consideration the structure and integrity of the visceral tissues lying beneath, two distinct, oval-shaped structures were seen to be placed directly beneath the two openings, firmly associated with with the dorsal body wall. These conspicuous, bright orange, and voluminous sac-like structures are the DAGs in the shape of inflated balloons whose diameter was larger than the orifices that directly release their secretions to the outside. The connection between the glands and the ostiole is retained via narrow ducts. Moreover, the glands are anchored by a rich supply of tracheal network. When one of the gland reservoir was pierced with a fine needle, a colourless, unscented and watery substance sprang out. In newly emerged adults the glands originally are large and inflated shown in Figure 2. However, as they reach their sexual maturity, the 4th tergal gland reservoir lost its structural integrity and are as tissue remnants that lay flatdorsal abdominal wall, while as the 5th gland retained its size and its natural architect (Figure 3).
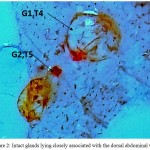 |
Figure 2: Intact glands lying closely associated with the dorsal abdominal wall.
|
While monitoring the changes in the morphology and development of DAGs throughout all stages, it was observed that as the insect advanced through the higher stages, the gland reservoir slightly changed its colour. It changed from dark reddish/orange to brown during latter part of the development as shown in Figure 3.
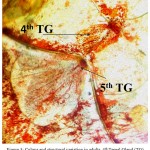 |
Figure 3: Colour and structural variation in adults. 4th Tergal Gland (TG) shown by arrow is dis structured
|
Body vs Gland Measurements
We plotted the largest diameter across the glands in every nymphal and adult stage against their corresponding body sizes. The two glands present in every stage are of almost equal size and, thus, the measurement of only one was recorded and plotted (Table 1). The adult females exhibited the largest body size (15.6±0.057 mm) whereas smallest size was recorded in the 1st nymphal instar i.e. 3.10±0.096 mm. The largest gland size was recorded in nymph 2 and, thereafter, it showed a declining trend as the insect progressed through rest of the nymphal and adult stages. Further, the highest ratio between the body and gland size was observed in females (11.81) followed by males (10.45). In nymphs, the ratio increased from 1st to the last instar. This relation can be easily depicted by means of a line graph (Figure 1).
Scanning Electron Microscopy
The scanning electron microscopic images provided further details and insight into the glands. The small, narrow, transverse openings called ostioles are present both in nymphs as well as adults; these ostioles aren’t ostensibly different from each other and are comparable structures. Through these ostioles the glands open to the outside medially, by means of ducts (Figure 5 G). In adults, honeycomb like cells i.e., sclerites are densely arranged around these orifices (Figure 4 F), and the peripheries of the ostioles appear to be rough and uneven. Moreover, the area surrounding the ostioles are associated with minute hair like projections that grow scantly around the openings (Figure 4 D). In nymphs, the ostioles are attached to fully functional glands beneath the integument they appear to be working factories in contrast to the adult ostioles where the 4th ostiole is just a sculpture of the non-functional gland. The area surrounded by the ostioles in nymphs appears to be smooth with no appearance of distinguishable sclerites (Figure 4 E). However, there are minute, rigid, and denticle-like structures (Figure 4 B) which represents the evaporatum.
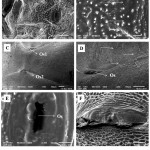 |
Figure 4: SEM images of the ostioles in nymphs (A, B and E) and adults (C, D and F), the structural variability can be compared in terms of ostiole (Os) opening (E and F) in nymphs and adults respectively and the structure of cells surrounding it.
|
The immediate peripheries are smooth in nymphs (E) and the adult ostioles are surrounded by honeycomb like cells (F) which have scanty growing hairs on them (D) indicated by arrows. Ridge like denticles(B) and the ostioles constitute the evaporation area in nymphs.
Structural Variation in Adult and Nymph Glands
Studying ontogeny by means of SEM through nymphs to adults, the pheromonal glands are observed to be attached to the terga 4th and 5th. In adults 5th tergal gland is fully structured (Figure 5B) whereas the gland placed beneath the 4th terga appears disordered seemingly lost its function (Figure 5C). Both the glands are extended into long ducts that connect them to their openings on 4th and 5th abdominal segments respectively (Figure 5D and G). The nymphs also possess the same architecture of the glands regarding location and size. The 4th and 5th tergal glands of all nymphal stages were found to be completely integrated, of same size, well-built and voluminous.
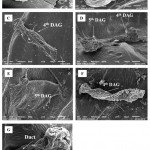 |
Figure 5: Scanning electron microscopy of the 4th and 5th Dorsal Abdominal Glands (DAG) dorsal view (A), (B and C) Magnified view of 4th and 5th DAG in adult.
|
Dorsal view of DAGs in nymph (D), shown separately in figure E and F. The duct indicated by the arrow connects the reservoir to the outside (G).
Discussion
The DAGs are invaginated cuticle-lined sacs that develop as metameric invaginated sacs that open inter segmentally, with exocrine gland cells forming their walls. Usually, there are three or four dorsal abdominal glands, the exact number and position being characteristic of a taxon (Cobben, 1978). More sophisticated, multicellular exocrine glands, with storage reservoirs from which secretion can be selectively released, have evolved in males of various terrestrial bugs. The diversity of the DAGs is vast. Owing to their diversity in terms of number and distribution, dorsal abdominal scent gland openings have also been used as an important phylogenetic tool (e.g. Putshkov 1958; Sweet and Slater 1961; Schuh and Slater 1995; Henry 1997, Gulde 1902; Dupuis 1947). Few studies have reported the morphology of the scent glands involving their ultra-structure and histological glandular preparations. The basic organisation and orientation of the glands remain almost similar all throughout the stages. Moreover, these glands are present right from the early instars asserting the fact that these may be involved in aggregation. With the advancement of age, the openings of these glands become concealed below the wings. Previously it was thought that there is a complete reduction of adult DAGs but subsequent studies have indicated the persistence of these glands in adults of heteroptera., and the present study substantiates the latter findings. Within pyrrhocoridae, D. cingulatus has been studied extensively for their exocrine secretions by Farine (1987), and he reported the presence of these glands on the 3rd, 4th, and 5th abdominal tergum which further consolidates our claim that DAGs are present on the 4th and 5th abdominal tergites with the same position and continuing throughout all the stages. Furthermore, gland vs body measurements revealed that the gland size do not vary much among the instars and between male and female. Similar results have been obtained by David G. James and Glen N. (1989) who reported that in adult stages the gland in Biproprorulus bibax breddin (Hemiptera: Pentatomidae) was reduced and became less functional, thus it can be concluded that their full functionality is seen in nymphal stages where they need to aggregate, probably, for better survival. Aldrich et al. (1978) concluded that in phytophagous species of hemipteran insects DAGs are small and of similar size in both the sexes with no sexual dimorphism further agreeing to our finding. Our study was the first attempt in the direction of elucidating the structural details of L. augur and various details remain obscure as of yet. Therefore, there is a need to dwell more in this field and try to reveal more about the structure of the gland and its associated structures, number and type of secretions, and age dependent changes, both in structure and function. Histological and analytical studies become a necessary thing to prove the functionality of these glands. The function of DAGs is complicated, attraction for the mate could just be a component of the complex functioning of DAGs. Last but not the least there is the need for ultimately putting all this information in the service of mankind by devising ways and methods for the control of pest insects.
Acknowledgement
The authors gratefully acknowledge University Sophisticated Instruments Facility, Aligarh Muslim University (USIF, AMU), Dr. Shahid Bin Zeya Associate Professor department of Zoology AMU for his kind support in helping us with the measurements, and UGC Non-Net fellowship for financial support.
References
- Dufour L. Recherches anatomiques et physiologiques sur les Hémipteres. — Mém Savants Étrang Acad. Sci. 1833;9:129-462.
- Kunkel M.J. Recherches sur les organs de se ´cretion chez les insects de l’ordre d’He ´mipte `res. Comptes Rendus de l’Acade ´mie des Sciences. 1866;120:1002–1004.
- valcurone D.M and pawan. M. Scent and defensive secretions of Rhyncota . pubblicazioni Dell’ instituto di entomologia Dell’ universita Di Pavia. 1978;51- 46.
- Staddon BW. The scent glands of Heteroptera. In: Advances in insect physiology. London: Academic Press. 1987;14:351–418.
- Staddon B.W. Biology of scent glands in the Hemiptera-Heteroptera. Annales de Socie ´te ´ Entomologique de France. 1986;22:183–190.
- Aldrich J.R, Blum M.S, Lloyd H.D and Falls H.M. 1978. Pentatomid natural products. Chemistry and morphology of the III-IV dorsal abdominal glands of adults. J. Chem. Ecol. 1978;4:161-172.
CrossRef - Aldrich J.R, Lusby W.R, Kochansky J.P and Abrams C.B. 1984. Volatile compounds from the predatory insect Podisus maculiventris (Pentatomidae): Male and female metathoracic scent gland and female dorsal abdominal gland secretions. Chem. Ecol. 1984;10:561-568.
CrossRef - Hamilton J.G.C, Gough A.J.E, Games D.E and Staddon B.W. Multichemical defense of plant bug, Hotea gambiae: (E)-2-Hexenol from abdominal gland in male adults. J. Chem. Ecol. 1985;11:1399–1409.
CrossRef - Ishiwatari T. Studies on the scent of stink bugs (Pentatomidae): I. Alarm pheromone activity. Entomol. Zool. 1974;9:153-158.
CrossRef - Lockwood J.A and Story R.N. Bifunctional pheromone in the first instar of the southern green stink bug, Nezara viridula: Its characterization and interaction with other stimuli. Entomol. Soc. Am. 1895;78:474-479.
CrossRef - Kou R, Tang D.S and CHOW Y.S. Alarm pheromone of pentatomid bug, Erthesina fullo (Hemiptera, Pentatomidae). J. Ecol. 1989;15:2695-2702.
- Remold H. 1962a. Scent-glands of land bugs, their physiology and biological function. Nature. 1992a;198: 764–768.
CrossRef - Remold, H. U ¨ ber die biologische Bedeutung der Duftdru ¨sen bei den Landwanzen (Geocorisae). Zeitschrift fu ¨r Vergleichende Physiologie. 1992;45:636–690.
CrossRef - Carayon, J. Note et documents sur l’appareil odorant me ´tathoracique des He ´mipte `res. Annales de Socie ´te ´ Entomologique de France (Nouvelle Se ´rie). 1971;7:737–770.
- Aldrich J.R, Yonke T.R, Oetting R.D. Histology and morphology of the abdominal scent apparatus in three alydids. Journal of the Kansas Entomological Society. 1972;45:162–171.
- Qadir I, Qamar A, Khan I Mir A .H and Begum R. A. brief review of Pentatomid Bug pheromones and their role in Integrated Pest Management (IPM). International Journal of Advanced Life Sciences. 2015;8:189-196.
- Bianchi F.M, Matesco V.C, Campos L.A and Grazia J. External morphology of the egg and the first and fifth instars of Cyrtocoris egeris Packauskas and Schaefer (Hemiptera: Heteroptera: Pentatomidae: Cyrtocorinae). Zootaxa. 2011;2991:29-34
- Pollo P, Greve C, Matesco V.C and Grazia J. Description of the immature stages of Glyphepomis spinosa Campos & Grazia (Hemiptera: Pentatomidae: Pentatominae: Carpocorini). Zootaxa. 2012;3566:61-68.
- GIlio-Dias S.M, Campos L.A and Bianchi F.M. Morphology of immatures of Caonabo pseudoscylax (Bergroth) (Hemiptera: Pentatomidae). Neotropical Entomology. 2013;42:178-184.
CrossRef - Cobben R. H. Evolutionary Trends in Heteroptera. Part II. Mouthpart-structures and Feeding Strategies. Veenman and Zonen, Wageningen. 1978; the Netherlands.
- Putshkov V.G. 1958 Larvae of Hemiptera-Heteroptera. I. Lygaeidae. Entomological Review. 1958;37:392–413.
- Slater J.A, Sweet M.H. A contribution to the higher classification of the Megalonotinae (Hemiptera: Lygaeidae). Annals of the Entomological Society of America. 1961:54:203–209.
CrossRef - Schuh R.T, Slater J.A. True bugs of the world (Hemiptera-Heteroptera). Cornell University Press, Ithaca (NY). 1995;336
- Henry T.J. Phylogenetic analysis of family groups within the infraorder Pentatomomorpha (Hemiptera: Heteroptera), with emphasis on the Lygaeoidea. Annals of the Entomological Society of America. 1997;90: 275–301.
CrossRef - Gulde J. Dorsaldru D. ¨sen der Larven der Hemiptera-Heteroptera. Bericht der Senckenbergischen Naturforschenden Gesellschaft in Frankfurt am Main. 1902;85–136.
- Dupuis C.D. ´es sur la morphologie des glandes dorso-abdominales des He ´mipte `reHe ´teropte `s. Feuille des Naturalistes. 1947;2:13–22.
- Farine J. P. 1987. The exocrine glands of Dysdercus cingulatus (Heteroptera, Pyrrhocoridae): Morphology and function of nymphal glands. Morphol. 1987;194:195-207.
CrossRef - David G, Glen J ,Warren N. Sexual Dimorphism of Dorsal Abdominal Glands in Biproprorulus Bibax Breddin (Hemiptera: Pentatomidae). Austral Entomology. 1989;28:75–76.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.






