Manuscript accepted on : 03 November 2017
Published online on: --
Synthesis of Bioactive Glass Powder using Sol-Gel Method and Shaping using Plasma Spark Sintering
Mehdi Mohsenpour Tehrani1 and Manocher Sobhani2
and Manocher Sobhani2
1Department of Technical and Engineering, Faculty of Materials and Ceramics, Shahroud Branch, IAU ( Islamic Azad University) Shahroud, Iran.
2Faculty of Material and Metallurgical Engineering, University of Semnan, Iran.
Corresponding Author E-mail: mahdipourtehrani96@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2600
ABSTRACT: In the present study, the synthesis of bioactive glass powder is carried out using the sol-gel method and in an acidic environment. TEOS (Tetraethyl orthosilicate) is used as the raw material of supplying Silica and TEP (three ethyl phosphates) is used as the raw material for providing phosphate powder compounds. Synthesis is done without water and using pure alcohol solvent 99.99%. After preparation of powder from the gel and drying it, the heat treatment process was performed at 500, 550 and 600 C for 2 hours on the powder. Thus, the bioactive glass powder was prepared for the SPS stage. In the present study due to technological limitations (impossibility of access to the high pressures of the SPS device used), at the formation stage, the maximum force of 20 tons was selected using the SPS device. Experiments performed on the powder include XRD and DTA. The results of this research show that performing heat treatment at a maximum temperature of 550°C with the aim of increasing the maintenance of the amorphous structure of the product is the most ideal temperature for heat treatment. Also, the use of suitable molds in the SPS process with a tolerance of up to 300 MPa is a requirement of the process.
KEYWORDS: Glass Powder Synthesis; Sol-Gel; Plasma Spark Plug; TEP XRD;
Download this article as:| Copy the following to cite this article: Tehrani M. M, Sobhani M. Synthesis of Bioactive Glass Powder using Sol-Gel Method and Shaping using Plasma Spark Sintering. Biosci Biotech Res Asia 2017;14(4). |
| Copy the following to cite this URL: Tehrani M. M, Sobhani M. Synthesis of Bioactive Glass Powder using Sol-Gel Method and Shaping using Plasma Spark Sintering. Biosci Biotech Res Asia 2017;14(4). Available from: https://www.biotech-asia.org/?p=28696 |
Introduction
The increasing number of bone problems during the last century due to world wars and other military conflicts, bone diseases, population growth and land transport followed by road accidents, sport injuries and a number of other external and genetic factors have pushed human to increase research and scientific studies on bones and bones implant over the past century. In this regard, research budgets are available to research centers on an annual basis from governments.
On the other hand, the average human life has increased due to the development of medical science and research in the field of drugs for treatment of serious illnesses. As a result, due to erosion of bone and joint tissues in the older ages, there are discomforts for humans that necessitate the development of science and technology in this regard.
In this regard, research is being carried out in the field of metal implants, metallic ceramic, ceramic and metal coatings with bioactive ceramic coatings in developed countries.
But one of the most important research areas in this regard is bioactive ceramics, due to the relative advantages of this group of ceramics in recent decades, much attention has been paid and much research has been done in this regard.
The advantages of bioactive- therapeutic ceramic include the raw material found in many mines in different countries, requiring less energy (compared to metal implants) to complete the production process, ease of the treatment process due to the permanence of the part grafted in the living tissue of the body and the repair condition of the tissue after the surgical procedure.
Biologically active glasses are among biologically active ceramics that the research on them began in 1969 at the University of Florida with the invention of the glass 45S5by melting method. This glass is a SiO2based and Na2O containing glass of four layers. But there are similar glasses that lack sodium in the composition of its raw materials and exhibit bioactive properties.
So far, the method of melting for the production of this group of ceramics has been able to be considered as an economic method for mass production. But due to the technological limitations of the melting method (in terms of equipment) and also the need for higher energy, researchers are looking for alternative methods.
One of these methods is the sol-gel method, which is a chemical-based approach. In this method, the initial stages of the production of bioactive glass powder are carried out at ambient temperature without the need for high temperature. Due to the fact that the reactions occur in molecular dimensions, powder particles are produced in the micro dimension and sometimes in the nano dimension, which also increases the bioactivity due to the increase of the specific surface area.
One of the advantages of this method in comparison with the melting method is to obtain a high homogeneous powder and a completely amorphous structure without impurities entering the system in the melting process.
Therefore in the present research using the experiments of other researchers it has been tried to synthetize powder using sol-gel method and then to form the powder using plasma spark plug.
The objectives of this research are as follows
Synthesis of bioactive glass powder by sol-gel method and control of hydrolysis and polycondensation reactions
Removal of Na2O in the compound by doing the process in lower temperatures using a triple composition of SiO2-CaO-P2O5
Estimation of the dependence of HA formation on the composition of glass (SiO₂ content in the glass composition)
Comparison of bioactivity between glass produced by melting method and Sol-gel glass
Methodology
TEOS (Tetraethyl orthosilicate) was used as the raw material for supplying silica and TEP (Three Ethyl Phosphate) was used as the raw material for supplying phosphate powder. Synthesis is done without water using pure alcohol solvent 99.99%. After powdering the gel and drying it, the heat treatment process was performed at 500, 550 and 600 ° C for h2 on the powder. Thus, the bioactive glass powder was prepared for the SPS stage. In the present study due to technological limitations (impossibility of access to the high pressures of the SPS device used), at the formation stage using SPS the maximum force of 20 tons was selected. Experiments performed on the powder include XRD and DTA.
For the preparation of quartz bioactive glass powder containing SiO₂-Na₂O-CaO-P₂O₅, which was first registered by Larry Hench, University of Florida in 1969, raw materials should first be selected. The acids that can be used to carry out the sol-gel process include HCl, HNO3, H2SO4.
Using SPS for Sintering
The operation starts by setting the process temperature, placing the powder inside the mold chamber and other settings. After closing the furnace door, the vacuum pump starts to create a vacuum in the compartment. Also, the moving arms of the device by introducing a vertical pressure to a mold up to MPa10 produces an initial drying strength in the powder. After creating a relative vacuum, the process of increasing the temperature of the unit begins. The rise of temperature occurs as the flow passes through the piece. In this way the temperature of the mold can rise up 50-100C as each 0.1 unit added the device. In this way, the speed control of the temperature is controlled by increasing the flow rate every 10 minutes. This process continues until the thermometer on the machine shows a temperature of about 750 ° C (100 ° C below the desired temperature). At this point, the temperature rise is interrupted and the maximum pressure of the device is applied. In the case of the device used, this pressure is MPa20. After removing the applied pressure, the machine is turned off and the piece is allowed to cool at an appropriate rate.1
Discussion
The sol-gel method has some advantages compared with melting method of bioactive glass production in terms of production facilities, the energy required and the time it takes to produce. Also, the initial investment cost in this method is lower than the melting method. In terms of glass produced by these two methods, it can be said that because of the high porosity and consequently the high level of glass produced in sol-gel, the bioactive properties of the powder are much higher. The sol-gel powder has less impurity due to pure raw materials, less unwanted combinations due to impossibility of reacting with ether and furnace walls at high temperature and better homogeneity of powder particles due to uniform mixing of the soluble phase and dry powder conversion and milling and heat treatment operations. The use of three-component glasses that does not contain sodium oxide makes it easier to use the sol-gel method. On the other hand the bioactivity of the three component sol-gel glass is higher than that of 45S5 glass produced by melting. Therefore if only the bioactivity parameter is considered and other parameters are neglected, we can use triple glass containing the raw materials SiO2, P2O5, CaO for the ease of process. The presence of compounds such as Na₂O in the glass network mainly accelerates the early stages of the formation of hydroxyapatite. Despite the replacement of Na₂O with oxides such as K₂O₅ and ZnO to achieve certain properties, the 45S5 is still considered to be the most bioactive type of glass.2 The presence of sodium compounds in the raw material due to its active ability can crystallize unwanted sodium compounds if it cannot control the hydrolysis and density of the reaction, which reduces the amorphous content of the powdered glass. Of course, in most studies, it has been pointed out that the crystallization temperature of the bioactive glass is about 550 ° C. Therefore, it is expected that under this temperature the crystalline phase will not crystallize. But in the quaternary glass containing Na₂O, the subject is slightly different. In these types of compounds, sodium at low-temperature syntheses can react with silicon and phosphate ions due to its high activity and form a crystalline structure. To prevent this, several factors must be monitored simultaneously. The first and most important control factor is the pH of the environment. The pH controls of the environment in the pH range of 1 to 2 are alkaline sodium and calcium cations. Indeed, as long as the H + concentration are high, the pH is maintained within the desired range. As soon as the H + ion concentration is reduced, it is possible for metal cations to react with the anions in the solution and to deposit. If the molar concentration of HNO3 ranges from 0.5 Mol to 1 Mol, fine particles are agglomerated and must be mechanically crushed in mass production. In contrast the powder particles obtained from 0.1 to 0.25 Mol HNO3 are spherical and finely divided and well separated. Therefore, the optimal concentration for acid is 0.1 to 0.25 Mol. In tables for combining with anions, the sodium ion wins and the precipitate is formed in solution. This deposition process is also a function of other variables.
Also the temperature of the process in the mixing step and gelatin has a direct effect on the time of gelatin and on formation of sediment at this stage. These parameters are factors that should be taken into consideration in the presence of sodium in the raw materials. But in triple glasses, the lack of sodium helps implement the process easily. The formation of the crystalline phase in the early stages of the sol-gel process makes the structure of the bioactive glass powder not completely amorphous and a percentage of the raw material is crystallized in crystalline phase. According to some researchers, this crystalline phase, which is generally a combination of calcium-sodium-silicate, can contribute to bioactivity. Because this phase, later and inside the body, is destroyed by SBF and forms HA. Therefore, for the precise control of the compounds in the bioactive glass, it is necessary to consider the optimum temperature of the process. Due to the fact that the mixing of raw materials is carried out in two phases which include the first phase of sodium and calcium dissolution in acid and in the second phase TEOS and TEP are dissolved in alcohol and then the two solutions are mixed together so it is necessary that the two solutions have the same temperature before they put adjacent to each other. Some other researchers also believe that as the structure of the bioactive glass is more amorphous, its bioactivity is higher. The XRD chart obtained from the bioactive glass powder heat treatment at 600 C indicates that in one stage of synthesis process which is probably the stage of producing sol or heat treatment, the crystalline phase of calcium- sodium- silicate is produced.
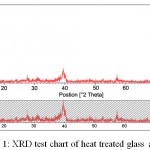 |
Figure 1: XRD test chart of heat treated glass at 600C
|
As shown in this diagram, the main peak seen in the diagram represents the crystalline phase. It is predicted that by controlling the effective factors in the sol-gel process, including temperature, acid concentration, and exposure time in the vicinity of the atmosphere and stirring speed, as well as control of the thermal operation cycle, it would be possible to prevent the formation of this phase to some extent. As mentioned in the previous chapters, the calcium-sodium-silicate phase can be formed at temperatures above 500 ° C. Therefore, in the process of heat treatment, we can expect to have a crystalline phase above this temperature.
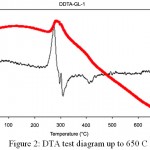 |
Figure 2: DTA test diagram up to 650C
|
According to the DTA diagram at 280C a heat peak is observed which is generally related to phase crystallization. Therefore, it is likely that part of the crystalline phase is crystallized at this temperature and the other part crystallizes at 630°C. On the other hand other samples were thermally heated at a temperature of 500C and in their XRD diagram the peak in 38C is observed. This means that the calcium-sodium-silicate phase is definitely crystallized below this temperature. It can be concluded that different phases of calcium- sodium – silicate can be crystallized at different temperatures which their reasons can be completely different. To eliminate hydrolyzed nitrates, it is necessary to heat up to 600°C. The compounds are reported to be harmful to living cells. On the other hand temperature up to 600C can form crystalline phase in 45S5 glass which leads to a decrease in bioactivity of the glass. Also, the high temperatures in the heat treatment can reduce the hydroxyl groups in the glass surface.4
Therefore, the maximum temperature for the heat treatment should be 640°C. Most of the waste and water compounds come out of the gel at a temperature of 550°C (Approximately 57% of 62% of total weight loss). This temperature is below 614°C, which is the crystallization temperature. Therefore, in order to prevent the formation of these phases and to increase the percentage of amorphousness of the synthesized bioactive glass powder, steps should be taken in the stage of sol-gel and in the heat treatment step [9]. After drying the gel that remains for a while at the temperature between 100-150C, a yellowish white powder remains which should be heat treated. A portion of powder particles are agglomerated during drying process of the gel. Therefore in order to ensure that the powder is fully stabilized and all powder particles are uniformly stable we should mill the powder by moony ruby or ball- mill before putting the powder in the furnace. This process causes that the powdered particles placed under heat treatment receive equal temperature and the structural water, dissolved water and nitrate compounds of these particles are uniformly removed during the heat treatment process. In the sol-gel method because the mixing of the particles of raw materials is done in molecular dimension, it is anticipated that the synthesized powder particles are in nano-scale and they are agglomerated during drying process. Therefore milling makes it possible for particles to reach micro and nano-dimensions and during the heat treatment process and the excess compound of the powder remove uniformly from it. It is better to use broad- groove bushes for powder heat treatment, because during the process, some of the volatile compounds are discharged into gas. These gases should be pulled out of the particle of powder and expelled to the surface of the powder. Therefore, during this time, the vapor pressure of these gases can throw solid powder particles out of the container. In order to prevent it, first the level of powder contact with furnace atmosphere should be increased and secondly use guard bushes. Research has shown that in sintering bioactive glass powder in a non- pressure condition, rapid crystallization occurs at a temperature between 580 and 650C which results in a crystalline solid at a lower temperature and prevents body condensation.5 Heat treatment at higher temperatures leads to the formation of Na2Ca2Si3O9 crystalline phase which results in the production of glass- ceramic.4 One of the glasses used is the 45S5 glass where 1 : the formation of Na2Ca2Si3O9 phase increases the mechanical strength.2 Crystallization process does not eliminate bioactivity and also with the ability to bone graft (the formation of hydroxyapatite phase) this ceramic still remains one of the ceramic with complete crystallization capability,3 When placed in the human body the crystalline phase Na2Ca2Si3O9 is decomposed and converted to the hydroxyapatite amorphous phase.2 One of the main problems in the sintering of 45S5 bioactive glass is that its rapid crystallization at high temperature which as a result due to the phenomenon of viscose flow it is prevented from its full density.2 The primary purpose of the heat treated gel sintering process is that sodium nitrate and calcium nitrate in the powder is decomposed and Na2O and CaO is obtained. The FTIR test confirms that the decomposition of the two NaNO3 and Ca (NO₃) ₂ compounds at 1000 ° C occurred completely.2 The complete decomposition of HNO3 occurs at 680C and complete decomposition of Ca (NO3)2 occur at 560 C and on the other hand the crystallization temperature of the bioactive glass 45S5 is 680 C. Therefore during the decomposition of HNO3 and Ca(NO3)2 crystallization occurs in this glass. However, the crystallization that happens cannot be worrying. Because the phase formed is Na₂Ca₂Si₃O9.2 On the other hand with increasing sintering temperature up to 600 c Na2CaSi2O6 phase is formed instead of Na2Ca2Si3O9 phase (which crystallizes in higher temperature).5 To prepare the powder a certain amount of it should be measured with the scales and cast into the mold. Due to the high porosity of the powder obtained from the sol-gel process, in the SPS process at high temperatures, there is the possibility of carbon penetration through the graphite strips used in the mold. Low temperature sintering is suitable for preventing carbon penetration into pieces of SPS graphite molds. From the comparison of different conditions of bioactive glass sintering by SPS, it can be concluded that the reason for reaching high density at a relatively low temperature of 500-550C in the present study can be due to low heating rate of 10 C/min compared to other methods with high heating rate of 100 C/min. In conventional sintering it is not possible to obtain amorphous samples with full density. Because the crystallization temperature of 600C is much lower than the temperature of full sintering which is 1000C.
After the powder has been prepared and the heat treatment is carried out to uniformize the powder and release the unwanted and unwanted waste that entered the system during the production process, it turns to the SPS process. Complete decomposition of HNO3 occurs at 680C and the complete decomposition of Ca(NO3)2 occurs at 560 C and on the other hand the crystallization temperature of bioactive glass 45S5 is 680C.4 It can be concluded that the developed method of SPS and bioactive glass sintering at 600 C can form the Na2Ca2Si3O6 phase which in comparison with the formation of Na2Ca2Si3O9 crystalline phase due to older methods of sintering or SPS at temperature below 600C, the presence of this phase in nanoscale dimensions can help hydroxyapatite formation speed on the surface of bioactive glass.4 As mentioned in the previous chapter the SPS device consists of a vacuum chamber in which fixed and movable molds are placed. This chamber is connected to a vacuum pump, which, after closing the enclosure door and sealing it, makes it possible to create a vacuum. The SPS chamber is equipped with a cooling system that is located next to the equipment. A metal thermometer and a pyrometer (optical thermometer) are also inside the enclosure. The metal thermometer should be placed at the nearest point inside the compartment to the piece. For this reason, according to the size and dimensions of the piece, it is possible to adjust its position. But the pyrometer is constant. The reason for using both of these devices to measure the temperature is that the metal thermometer can operate up to 800°C, and for higher temperatures the pyrometer should be used. On the other hand, the pyrometer can detect temperatures above 1000°C. This is one of the problems with using the SPS device in the Materials and Energy Research Institute. Also there is always a temperature difference between outside temperature of the piece and its internal temperature which cannot be measured. Therefore usually between 50 and 100 C tolerance is considered by the operator of the device. But in a study conducted by three researchers, Salvatore Grasu, Yoshio Saka and Giovanni Maisa,6 statistical surveys were carried out on various temperatures and pressures of the SPS system.
In accordance with Figures 3 and 4, the difference in temperature between the outer and inner surfaces of the die decreases with increasing pressure relative to time and temperature. Therefore, if the pressure used in the SPS process increases, the temperature difference between the outside and inside of the die decreases. At a pressure of MPa80, at 700°C this temperature difference will reach 50°C. This means that at higher pressures, the temperature can be controlled more accurately.
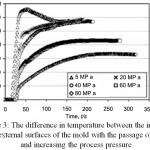 |
Figure 3: The difference in temperature between the internal and external surfaces of the mold with the passage of time and increasing the process pressure
|
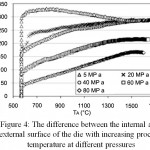 |
Figure 4: The difference between the internal and external surface of the die with increasing process temperature at different pressures
|
On the other hand in order to perform sintering process on the bioactive glass powder, it should be noted that in accordance with DTA test charts at temperature above 640C, a calcium- sodium- silicate phase is formed which is stable up to 1600C. The crystallization temperature of the bioactive 45S5 glass has been reported at 610, 650, and 690°C.4 This means that with the process temperature rising, there is a probability that this phase will be formed. Therefore in order to avoid phase formation at this temperature, process temperature control is necessary to the below the temperature of the phase formation. Also in order to achieve the desired strength at a low temperature, the process pressure should be increased. In a study, this pressure has increased from 70 to 300 MPa. Under these pressures, the molds of the device should be able to withstand applied stresses.3 The SPS device in the Materials Research Institute has graphite molds which are used mainly for high temperature powder sintering at low pressures. The process was carried out for the first time at 550C and a maximum pressure of 20 megaPascal. From the beginning of the process up to a temperature of 550°C, the pressure is maintained at 10 MPa and after reaching 550°C, the force increased to 20 tons and remained constant for 5 minutes. Then the pressure was removed and the temperature, which increased at a rate of 5°C per minute, decreased by 10°C per minute.
The rate of decrease and increase of temperature is a function of the flow through the mold and piece. Therefore, the temperature can be controlled by adjusting the flow. This is done empirically by the operator in the SPS device in the Research Institute. In order to prevent the increase of unwanted temperature in the final stages of the process, the temperature rise rate should be reduced. The effect of this rate reduction is that the temperature is distributed uniformly throughout the body, resulting in high density at lower temperatures.5 Due to the low temperature and pressure used in the process, after sintering the intended part does not reach to the required density. Therefore, the mechanical strength of the piece cannot be expected. It was therefore decided to repeat SPS process for synthesized powder once more by applying higher temperature. As mentioned in the previous chapter, and in accordance with the Grasso et al., (2009),6 due to the impossibility of applying pressure higher than MPa20 on the SPS device, we had to use a higher temperature to approach the density of the theory around 800°C.
Suggestions
According to studies carried out on previous research and experiments carried out during the project, the following suggestions are provided for future research:
Most researchers who have done researches with the aim of bioactive glass synthesis by sol-gel method have used acidic environments. It is likely that alkaline environment has advantages compared with acidic environment. Therefore the subject of this synthesis in alkaline environment and comparison with acidic environment can be considered as a research.
Choosing the type and the ratio of acid to other basic liquid substances is very important for acidic environment.
The process of synthesis with and without water is a topic that can be studied. The presence of water can cause some of the solid phases to precipitate during the gelatin stage. On the other hand, the presence of H and OH ions in the water after entering the system can be economical.
The appropriate time for the gel aging stage can be considered as a topic for the study of the synthesis of bioactive glass.
The effect of pH changes on the phases formed in the sol-gel stage is a significant parameter.
The use of raw materials that does not have harmful elements for the human body and the replacement of the common materials used in research is a subject that can be investigated.
Performing the SPS process at lower temperatures with higher pressure and also at higher temperature with lower pressures are subjects that should be examined more closely. What happens after the SPS process at high temperatures, what phases it may take, and how it affects the amount of bioactivity of the synthesized glass.
The use of metal molds with the ability to transfer electrical current and its comparison with graphite molds is an important subject that needs to be carefully examined. Also in the SPS process, the internal temperature and the temperature of the exterior surface of the body within the mold are different. And this difference is not predictable due to the impossibility of determining the temperature created in the unit due to spills discharged in the piece. On the other hand, the structure, composition, and final strength of the body inside the SPS device owe the control of the temperature inside the compartment. Research in this regard is required.
Performing the bioactivity test of various SPS samples at different temperatures and pressures and comparing their bioactivity.
Test the strength of sintered piece before and after putting in SBF solution and investigate the effect of formation of hydroxyapatite phase on the strength of the component.
Conflict of Interest
We have no conflict of interest to declare.
References
- Suárez M, Fernández A, Menéndez J.L, Torrecillas R, Kessel H.U, Hennicke J, Kirchner R and Kessel T. Challenges and Opportunities forSpark Plasma Sintering:A Key Technology for.a New Generation of Materials, doi:10.5772/53706.
CrossRef - Suárez M, Fernández A, Menéndez J.L, Torrecillas R, Kessel H.U, Hennicke J, Kirchner R and Kessel T. Challenges and Opportunities forSpark Plasma Sintering:A Key Technology for.a New Generation of Materials, doi:10.5772/53706.
CrossRef - Qi-Zhi C, Li Y, Li-Yu J,Julian M.W.Q,Paul A. Komesaroff A new sol–gel process for producing Na₂O-containing bioactive glass ceramics. Acta Materialia Inc. Published by Elsevier Ltd. 2010.(doi:10.1016/j.actbio.2010.04.022).
- Rezabeigi E,Paula M, Wood-Adams,Robin A.L. Drew Department of Mechanical and Industrial Engineering, Concordia University,Montreal,QC H3G 1M8,Canada.Synthesis of 45S5 Bioglass® via a straightforward organic,nitrate-free sol-gel process, doi:10.1016/j.msec, /2014. Elsevior BV All right reserved. 2014;03(042):0928-4931.
- Grasso S, Krishna R.C, Porwal H, Aldo R.B, Michael J. Reece Low temperature spark plasma sintering of 45S5 Bioglass®. 2012 Elsevier B.V. doi:10.1016/j.jnoncrysol. 2012;11:009.
CrossRef - Grasso S, Sakka Y, Maizza G. Pressure Effects on temperature tistribution during Spark Plasma Sintering with Graphite Sample. Materials Transactions. 2009;50(8):2111 to 2114, 2009.The Japan Institute of Metals.
- Li R,Clark A.E and Hench L.L. Advanced Materials Research Center, College of Dentistry, University of Florida,An Investigation of Bioactive Glass Powders by Sol-Gel Processing. Journal of applied biomaterials. 1991;2:231-239.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





