Manuscript accepted on : 25 December 2017
Published online on: --
Hasanali Mollashahi1 , Ali Mirshekari1
, Ali Mirshekari1 , Morteza Ghorbani1
, Morteza Ghorbani1  and Armin Tarrah2
and Armin Tarrah2
1Department of Plant Protection, University of Zabol, Iran.
2Department of Agronomy Food Natural Resources Animal and Environment (DAFNAE), University of Padova, Italy.
Corresponding Author E-mail: armin.tarrah@phd.unipd.it
DOI : http://dx.doi.org/10.13005/bbra/2571
ABSTRACT: Chrotogonus trachypterus Blanchard is an active grasshopper on farms in Sistan and Baluchestan province, Iran, in high populations and is one of the most important pests of vegetables, alfalfa, early growth stages of Gramineae and many crops. Chemical pesticides have harmful effects on the environment and human health; therefore, the use of natural insecticides derived from plants play significant role in pest management because of lower costs, lack of environmental pollution and protection of human health. In this study, lethal effect of Citrullus colocynthis fruit extract was evaluated on the Ch. trachypterus grasshopper under controlled laboratory conditions (at a temperature of 24±2°C, relative humidity of 75±5%, and photoperiod of 16: 8) in a completely randomized design with three replications. The plant was collected from its natural habitat around the city of Iranshahr, Iran, and then dried in the shade. The extraction was performed using methanol. The results obtained from experiments showed significant increase in mortality of tested insects after 24 hours with increasing concentrations of plant extracts. The effect of this extract with concentrations of 10, 20, 25, 35 and 40 milligrams per milliliter were tested on adult Ch. trachypterus grasshoppers. The highest mortality rate (87.50%) was found at a concentration of 40 milligrams per milliliter and the lowest mortality rate (23.33%) was observed at concentrations of 10 milligrams per milliliter. The mortality rate elevated with increasing concentration in all treatments. The LC 50 value for C. colocynthis plant on adult grasshopper was calculated 18.58 milligrams per milliliter.
KEYWORDS: Chrotogonus Trachypterus; Citrullus Colocynthis; Herbal Extract Plant Insectisides;
Download this article as:| Copy the following to cite this article: Mollashahi H, Mirshekari A, Ghorbani M, Tarrah A. Insecticidal Effect of the fruit Extract Bitter Melon (Citrullus Colocynthis) on Locust Chrotogonus Trachypterus (Orth: Pyrgomorphidae). Biosci Biotech Res Asia 2017;14(4). |
| Copy the following to cite this URL: Mollashahi H, Mirshekari A, Ghorbani M, Tarrah A. Insecticidal Effect of the fruit Extract Bitter Melon (Citrullus Colocynthis) on Locust Chrotogonus Trachypterus (Orth: Pyrgomorphidae). Biosci Biotech Res Asia 2017;14(4). Available from: https://www.biotech-asia.org/?p=28534 |
Introduction
Grasshoppers have always been raised as one of the most important pests of crops in all agroecosystems; heavy and irreparable damages caused by their feeding have driven man to look for appropriate way to control these dangerous insects. The history of using herbal ingredients as insecticide goes back at least 400 years BC to the times of Roman Empire, which the powder of dried flowers of pyrethrum (Chrysanthemum cincriaefolium) was used for the first time. Plinus (23-79 BC) described the use of ash and mashed leaves of cypress tree (Cupressus spp.) in controlling insects (Alder et al., 2000). Plants over millions of years through natural selection during evolution have been equipped with various compounds against different pests, which will protect them against the attacks of varied enemies as a weapon. Moreover, many of these compounds have no harmful effects of synthetic compounds of pesticides on humans and the environment (Enan, 2001).
Bitter melon or colocynth (Citrullus colocynthis schard. Fam. Cucurbitaceae) belongs to the Cucurbitaceae family that contains compounds of cucurbitacins; the cucurbitacins in the bitter melon are in the category of steroidal saponins (Natio et al., 1989). Bitter melon fruit is used to treat constipation (Abdel-Hassan et al., 2000), diabetes mellitus (Grover et al., 2002), rheumatic diseases, skin infections (Tannin-Spitz et al., 2007) and sore throat (Elawad et al., 1984). This plant has been utilized in Iranian traditional medicine in the treatment of constipation, bacterial infections, cancer and diabetes as well as for abortion (Madari and Jacobs, 2004).
These compounds, which are known as secondary metabolites, are essential for the survival of plant cells but are useful for the reaction of organisms against the environment. These compounds have major role in plant defense against herbivorous insects and act as repellent, nutritional deterrent, spawning or toxic compounds, and protect the plants against insects in different growth stages. Given the inevitability of human in the use of pesticides, botanical insecticides could be partly alternative to synthetic insecticides due to features such as minimal impact on natural enemies, lack of phytotoxicity, minimum toxicity on vertebrate and rapid degradation in the environment that is one of the most important advantages of this type of insecticides (Isman, 1997). So far, many studies have been carried out in connection with insecticidal properties of plant products.
In this study, the effect of bitter melon fruit extract was investigated on a species of grasshopper native to Sistan. Given the diversity of climate in our country, we hope to identify herbal effective compounds in the field of pest control, and in the not too distant future to produce and use new substances and low-risk pesticides to humans and environment.
Materials and Methods
Collection of Study Plants
Bitter melon, Citrullus colocynthis (Cucurbitaceae), was the study plant that was collected from natural habitats during March to July 2012 in Sistan and Baluchestan province, the city of Iranshahr. The fruits were separated from plant and dried at a temperature of 23-24°C in the shade. The seeds were separated from the fruit and other parts were stored in a paper bag in the refrigerator.
Preparation of Methanol Extract
The methanol 96% was used as a solvent. According to Elbadri et al. in 2008, the dried fruit was powdered using household electric grinders. Then, 100 grams of powder was weighed and extracted twice and each time with 500 ml of methanol 96% for 24 hours on a shaker with 150 rpm according to Bahraminejad et al., 2008. Containers used for extraction were covered with aluminum foil to prevent the exposure of light (Mahdavi Arab et al., 2007). After each extraction, the extract was filtered using Whatman filter paper No. 1 and the solvent was finally evaporated from the obtained solution using rotary evaporator. The extracts were stored in dark glass container until use.
Determination of Dry Weight of Methanol Extract
First, empty petri dishes were weighed, and then 5 ml of the filtered extract was poured in some petri dishes. The petri dishes were placed in an oven at a temperature of 100°C-105°C to dry completely their contents. The petri dishes containing dried extract were weighed. The weight of dried extract was determined by their difference with the weight of the empty petri dishes. The dried extract contained in one milliliter was calculated. The mean of three replications was considered as the weight of dried extract. Thus, the extract concentration can be expressed in terms of ppm (Ghaemi et al., 2006).
Collection of Insects and Breeding in Lab
The insects needed for the research were obtained from Agriculture and Natural Resources Research Center of Sistan and delivered to the laboratory of Entomology, Department of Plant Protection, Faculty of Agriculture, University of Zabol. Breeding was performed under controlled laboratory conditions at a temperature of 30±5°C, relative humidity of 40±5%, photoperiod of 16 hours of light and 10 hours of darkness. To breed adult insects, wooden cages with dimensions of 35×35×35 cm were recruited. The cages were coated by wire mesh on three sides and floor and by glass from the front. In each cage, some cavities were applied to put oviparity cylinders. To provide heat radiation, a lamp was mounted in cage outside and was connected to a timer for adjusting the lighting on and off. For oviposition, some sands after washing and disinfecting were poured into 110-cm3 aluminum cylinders, and then kept wet with 15 to 35 ml of distilled water to provide adequate moisture at the site of oviposition. The cylinders were visited every day to replace with new cylinder by observing the egg capsules of grasshopper. After forming the egg capsules, oviparity cylinder duct was blocked by aluminum foil and was put in an incubator at a temperature of 35°C inside the Plexiglas containers with mesh floor and cap. To breed nymphs, PVC transparent cylindrical containers with wire mesh lid and floor were used. The adult insects and nymphs were fed daily by alfalfa, lettuce and basil after disinfection (Lal-Jat et al., 2007; Syed, et al., 2011).
Lethal concentration of 50% (LC50) of herbal extract
Lethal concentration of 50% (LC50) of the plant extract and five concentrations of the extracts leading to mortality between 20 and 80 percent were investigated in this study with three replicates for each concentration. Ten seven-day adult insects were used for each treatment. Topical application method was employed to infect the insects (Mc caffery et al., 1988; Dahm et al., 1968), so that one microliter of a concentration was placed under pronotum of grasshopper by a sampler. Only distilled water was used for control treatment.
Statistical Analysis
Testing was conducted in a completely randomized design with three replications. Original experimental numbers have been inserted in the tables. If there were significant difference, the means would have statistically compared using the Tukey’s test at 5% level. Statistical analyzes were performed using SPSS 16 software and drawing graphs in Excel 2003.
Results and Discussion
The LC50 calculated for study insects 24 hours after applying the extract showed that the LC 50 value for C. colocynthis effect on Ch. trachypterus was calculated 18.58 milligrams per milliliter. The mortality rate after 24 hours elevated significantly at the level of one percent with increasing concentrations of methanol extract of bitter melon fruit (F4, 10 = 14/55, P = 0).
Table 1: Lethal concentration of 50% with 95% confidence level and chi square of C. colocynthis extract
| sex | Number | LC50 | Lower limit | Upper limit | Chi square (df) | Intercept±SE |
| (M,F) | 160 | 18.58 | 14.70 | 21.98 | 0.908(3) | 2.94±0.52 |
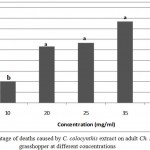 |
Figure 1: Percentage of deaths caused by C. colocynthis extract on adult Ch. trachypterus grasshopper at different concentrations
|
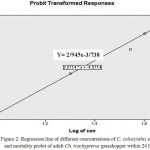 |
Figure 2: Regression line of different concentrations of C. colocynthis extract and mortality probit of adult Ch. trachypterus grasshopper within 24 hours
|
In evaluating the effect of plant extract of bitter melon (Citrullus colocynthis) on the Ch. trachypterus grasshopper revealed that the extract has insecticidal effect and the mortality rate rises with increasing concentrations and with the passage of time. Other studies also show the sensitivity difference of the insect and the plant lethal power in different concentrations (Torkey et al., 2009; Abdul Rahman and Venkatesan, 2008). Mullai and Jebanesan in 2007 examined the effect of bitter melon extract against the mosquito of Culex quinquefasciatus (Dip: Culicidae). The results suggested that bitter melon extract has toxic and lethal effects on the insect studied and mortality enhanced with increasing concentration. The results of Rawi et al., 2011 indicate that the methylene chloride extract of bitter melon has insecticidal effect on larvae of Spodoptera littoralis. According to the results of this research and other studies, it can be concluded that the Chrotogonus trachypterus grasshopper has reasonable sensitivity to bitter melon extract. This plant extract can be introduced as an alternative to conventional synthetic pesticides against the mentioned pests. It should be noted that further scientific studies in biochemistry and technical levels are required to use of the plant compounds and formulating them.
References
- Abdel-Hassan I. A, Abdel-barry J. A And Tariq-Mohammedas. The hypoglycaemic and antihypoglycaemic effect of Citrullus colocynthis fruit aqueous extract in normal and alloxan diabetic rabbits. Journal of Ethnopharmacology. 2000;71:325-330.
CrossRef - Rahuman A.A and Venkatesan. Mosquito larvicidal activity of oleic and linoleic acids isolated from Citrullus colocynthis schrad. Parasitol Research. 2008;103:1383-1390.
CrossRef - Alder C,ojimelukwe p and Tapondjou A.L. Utilisation of phytochemicals against stored product insects.Integrated protection of stored products. 2000;23(10):169-175.
- Bahraminejad S, Asenstorfer R. E, Riley I. T and Schultz C.J. Analysis of the antimicrobial activity of flavonoids and saponins isolated from the shoots of oats (Avena sativa L.). Phytopathology. 2008;156:1-7.
- Dahm P.A, Garland J, Lee I and Berlin J. A comparison of some housefly bioassay methods. Journal of Economical Entomology. 1968;54:343- 347.
CrossRef - Elawad A. A, Abdel-Bari E.M, Mahmoud O.M and Adam S.E. The effect of Citrullus colocynthis on sheep. Vet Hum Toxicol. 1984;26(6):481-485.
- Elbadri G. A, Lee D.W, Park J.C, Yu H.B and Choo H.Y. Evaluation of Varius Plant Extracts for their nematicidal efficacios against Juveniles of Meloidogyne incognita. Journal of Asia-pacific Entomology. 2008;11:99-102.
CrossRef - Enan E. Insecticidal activity of essential oil octapaminergic sites of action comparative. Biochimestry and physiology. 2001;130-323.
- Ghaemi A, Jahy S.H, Moghaddam F.M, Yazdani N And Dizaji Z.H. Evaluation of antiviral activity in the control of HSV Purple coneflower extract vegetative body of a human. Hakim Research Journal. 1385;9(4):59-64.
- Grover J.K, Yadav S and Vats V. Medical plants of India with anti-diabetic potential. Journal of Ethanopharmacology. 2002;81:81-100.
CrossRef - Isman M.B. Neem and other botanical insecticides. borriers to commercialization. phytoparasitica. 1997;25(4):339-344.
CrossRef - Lal-Jat S, Swaminathan R and Rathore P.S. Biological studies on the surface Grasshopper, Chrotogonus trachypterus (Blanchard) at Udaipur. International Journal of Tropical Agriculture. 2007;25(3): 681-688.
- Madari H and Jacobs R. S. An analysis of cytotoxic botanical formulations used in the traditional medicine of ancient Persia as abortifacient. J. Nat. Prods. 2004;67:1204-1210.
CrossRef - Arab M.N, Ebadi R, Hatami b And Jahromi T.K.H. Insecticidal effect of some plant extracts on Callosobruchus maculatus and Laphigma exigua on in the laboratory and greenhouse. Science and Technology of Agriculture and Natural Resources. 1386;11(42):221-234.
- Mccaffery A.R, Marut, Walker A.J and Styles k. Resistance to pyrethroids in Heliothis spp: Bioassay Methods and Incidence in Population from India and Asia. 1988;433-438.
- Mullai K and Jebanesan A. Larvicidal, ovicidal and repellent activities of the leaf extract of two cucurbitaceous plants against filaria vector Culex quinquefasciatus (Say) (Diptera: Culicidae). Journal of Tropical Biomedicine. 2007;24(1):1-6.
- Natio A. R, Hatam-Donald A, Whiting N and Yousif J. Cucurbitacin Glycosides from. Citrullus colocynthis. Phytochemistry. 1989;28(4):68-71.
- Rawi S. M, Bakry F.A and Al-Hazmi M. A. Biochemical and histopathological effect of crude extracts on Spodoptera littoralis larvae. Journal of Evolutionary Biology Research. 3(5):67-78.
- Syed T.S, Lal M, Abro G.H and Siddiqui S. Effect of consumption of different hosts on the body weight of surface grasshopper, Chrotogonus trachypterus Blanchard (Orthoptera: Pyrgomorphidae). Sarhad. J. Agric. 27(2):245-249.
- Tannin-Spitz T, Grossman S, Dovrat S, Gottlieb H.E and Bergman M. Growth inhibitory activity of cucurbitacin glucosides isolated from Citrullus colocynthis on human breast cancer cells. Journal of Biochemistry Pharmacology. 73(1):56-67.
- Torkey H. M, Abou-Yousef H. M, Abdel Azeiz A. Z and Hoda E. A. Farid. 2009. Insecticidal effect of cucurbitacin E Glycoside isolated from Citrullus colocynthis against Aphis craccivora. Australian Journal of Basic and Applied Sciences. 3(4):4060-4066.

This work is licensed under a Creative Commons Attribution 4.0 International License.





