Manuscript accepted on : 06 November 2017
Published online on: --
Cell Surface Hydrophobicity as A Virulence Factor in Candida Albicans
Renuka R. Goswami1, Suhas D. Pohare1, Jayant S. Raut1, 2 and S. Mohan Karuppayil1
and S. Mohan Karuppayil1
1DST-FIST and UGC-SAP sponsored School of Life Sciences, SRTM University, Nanded, 431606, (MS), India.
2University Institute of Pharmaceutical Sciences (UIPS), UGC Center of Advanced Study, Panjab University, Chandigarh, 160 014, India.
Corresponding Author E-mail: prof.karuppayil@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2598
ABSTRACT: Cell surface hydrophobicity (CSH) is one of the important virulence attributes which helps Candida albicans to be a successful fungal pathogen. It influences several steps in pathogenesis of C. albicans leading to establishment of infection. CSH plays an important role in adhesion of cells to host tissues and catheters/medical devices implanted in patients. Adhesion to surfaces and subsequent biofilm formation are crucial because it may result in resistance to antifungal drugs. This important pathogenicity determinant would also be an attractive antifungal target. Various studies indicate that antifungal drugs tend to lower the CSH of Candida cells. Interestingly, molecules of plant origin have been reported to modulate CSH, reduce adhesion and interfere in biofilm formation by C. albicans. The review presents a brief account of biochemical basis of CSH, its role in adhesion and biofilm formation by C. albicans as well as explores it as an antifungal drug target.
KEYWORDS: Adhesion; Biofilm; Candida Albicans; Cell Surface Hydrophobicity; Drug Target; Virulence
Download this article as:| Copy the following to cite this article: Goswami R. R, Pohare S. D, Raut J, S. Karuppayil S. M. Cell Surface Hydrophobicity as A Virulence Factor in Candida Albicans. Biosci Biotech Res Asia 2017;14(4). |
| Copy the following to cite this URL: Goswami R. R, Pohare S. D, Raut J, S. Karuppayil S. M. Cell Surface Hydrophobicity as A Virulence Factor in Candida Albicans. Biosci Biotech Res Asia 2017;14(4). Available from: https://www.biotech-asia.org/?p=28218 |
Introduction
Commensal yeasts like Candida species inhabit oral cavity, gastrointestinal tract, urino-genital regions and skin surface of the humans.1 Among the Candida species, C. albicans is the most frequently isolated from patients and has been considered as a model for studies on human pathogenic fungi.2 This fungus which is harmless in healthy individuals may turn pathogenic in people with compromised immune status.3 The immunocompromised population susceptible to C. albicans infections include cancer patients, AIDS patients, patients under long term steroidal treatment, chemotherapy or prolonged use of antibiotics, patients under intensive care and persons who have undergone organ transplantation or surgeries, diabetic people, new born, old age people, and patients using prosthetic devices/ medical implants.4 Infections caused by C. albicans range from superficial infections to systemic diseases. Systemic candidiasis involves blood stream infections, deep seated tissue infections of heart, kidney, liver, spleen and brain.3 Moreover, C. albicans readily colonizes prosthetic devices implanted in the patient’s body to cause device associated biofilm infections.5,6 It is estimated that around 90% of the HIV infected persons experience candidiasis once in their survival period.7 At least 15% of the immunocompromised people develop a systemic illness due to Candida which may be associated with 75% of death rate.8 Candida albicans is the fourth most common cause of hospital acquired infections and ranks third in catheter related infections in the United States of America.9 The treatment cost associated with Candida infections is estimated to be more than US $ 1 billion per annum.8
Various virulence attributes help pathogenic behavior of C. albicans. Some of the important virulence factors are morphogenesis, adhesion, surface virulence molecules (receptors, adhesins, and immune modulators), lytic enzymes (e.g. secreted aspartyl proteases and lipases) and biofilm formation.2,10 Expression of a specific virulence factor by C. albicans depends on the site of infection, growth stage and also on the host response. A small variation in virulence factor may cause C. albicans to shift from commensal to pathogen and vice-versa.11 Enhanced virulence of C. albicans is found to be associated with an important factor i.e. cell surface hydrophobicity (CSH).12 The CSH influences several steps in pathogenesis such as, nonspecific (other than ligand-receptor mediated) adhesion to host tissues and implanted medical devices, enhanced germ tube induction, increased co-aggregation and colonization of cells, biofilm formation, avoidance of neutrophil mediated killing and protection from host defense mechanisms.13,14,15 This review takes an account of biochemical basis of CSH in C. albicans, factors responsible for its variation, CSH as a virulence determinant and explores it as an antifungal drug target.
Biochemical Basis of Csh
Under different environmental and growth conditions C. albicans cells can exist as either hydrophilic (water interactive) or hydrophobic cells (less water interactive). Hydrophobic cells are considered to be more virulent than hydrophilic cells.16 Comparative analysis of these two forms of cells indicated that hydrophilic and hydrophobic cells are biochemically similar, but ultrastructure of their cell walls is different.17 The cell wall of C. albicans is a complex structure made up of polysaccharides (80-90%), proteins (5-15%) and lipids (1-7%).18 Three basic subunits of cell wall polysaccharide are β-glucan, chitin and glycoprotein. β-glucan which constitutes 47 to 60% part of the polysaccharide is a branched polymer of glucose with β- 1, 3 and β- 1, 6 linkages. Chitin is an unbranched polymer of N- acetyl-D- glucosamine containing β-1, 4 linkage and have 6-9% share in the polysaccharide chain. While, the mannoproteins which are polymers of mannose (mannan) covalently associated with cell wall proteins constitute up to 40 % part of the cell wall polysaccharide.19 The CSH of C. albicans is found to be susceptible to various protease treatments indicating that surface hydrophobicity is due to presence of proteins. Enzymatic digestion is required to remove hydrophobic cell wall proteins which suggested that the hydrophobic proteins are tightly associated or covalently linked to the cell wall matrix.20 Various proteins are extracted from the cell wall of C. albicans, from which hydrophobic proteins (hydrophobins) are specifically separated and analyzed.18 Most of the identified hydrophobic proteins are in the range of 34 to 60 kDa. For example, a 34-kDa β- glucan branching enzyme, 37-kDa laminin binding protein, 38-kDa Csh1p protein, 40-kDa alcohol dehydrogenase, 41-kDa lysophospholipase and 58-kDa fibrinogen binding protein are some of the characterized hydrophobic cell wall proteins.19,21-23 Among all the hydrophobic proteins, Csh1p is found to be the main protein contributing to hydrophobicity of C. albicans cell surface.
Freeze- fracture analysis of the cell surface of C. albicans have revealed that hydrophilic cell surface possess long, densely packed and evenly spaced fibrils on the cell wall. Whereas, the hydrophobic cell surface display short, evenly spaced and blunt fibrils.18 The presence of similar hydrophobic proteins on both i.e. hydrophilic and hydrophobic yeast cell surfaces indicated that there is no qualitative difference in proteins which determine these two types. The only difference is that proteins contributing to the hydrophobic behavior are not readily exposed at the surface in hydrophilic cells.18 This is because of the existence of high- molecular weight mannoprotein fibrils which mask the hydrophobic proteins on the cell surface of hydrophilic cells.22
Simple loss or attenuation of the outer fibrillar layer of mannoproteins from hydrophilic cells is found to result in exposure of hydrophobic proteins on the cell surface.22 Cell surface hydrophobicity (CSH) of C. albicans is thus due to direct contribution of various surface hydrophobic proteins and the indirect contribution of mannosylated glycoproteins which form ectomural fibrils.14,24 The cell wall fibrillar mannoprotein consists of mannose polymer linked to the protein through asparagines by N-linked glycosidic bond. The linked carbohydrate is composed of chains of acid-stable α-1, 6 linked mannose and α-1, 2 linked mannose,19 as well as an acid-labile β-1, 2 linked mannose. The acid labile branch is attached to the α-1, 2 mannan chain via a phosphodiester bond.25 There is no overall difference in protein, hexose or phosphate composition of mannoproteins extracted from hydrophilic and hydrophobic yeast cells. Only significant difference is the acid-labile β-1, 2 oligomannosyl branching in mannoprotein.16,26
Acid labile mannan with β-1, 2 linkages in hydrophobic cells contains a longer chain of oligosaccharides than that of hydrophilic cells.25,26 Computer models proposed that increase in the chain length of β-1, 2 oligomannan in hydrophobic cells cause an inter-fibril interaction that leads to fibril folding, aggregation, and inflexible helix formation.16
The presence of long chain of β-1, 2 linked mannose cause inter-fibril folding and aggregation. As a result these fibrils tend to be short and blunt which cannot mask the hydrophobic proteins present on the surface of hydrophobic cells. Short chains of acid-labile β-1, 2 linked mannose are incapable of inter-fibril interactions and folding of fibrils can’t occur; hence, the long, densely packed and evenly spaced fibrils mask the hydrophobic proteins on the cell surface in hydrophilic cells (Figure 1).15,18
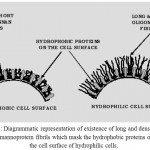 |
Figure 1: Diagrammatic representation of existence of long and dense layer of mannoprotein fibrils which mask the hydrophobic proteins on the cell surface of hydrophilic cells. |
Regulation of a single step in cell wall protein glycosylation i.e. elongation of the acid-labile β-1, 2 oligomannoside is sufficient to regulate the switch between hydrophilicity and hydrophobicity of C. albicans cell surface.16
Factors Influencing Csh
Various physicochemical and biological factors influence cell surface hydrophobicity of C. albicans.27 CSH is shown to undergo significant changes within minutes of C. albicans exposure to new environmental conditions.12 For example, growth temperature influences the relative CSH level inversely. It is high at 23˚C and decreases when growth temperature increases from 23˚C to 37˚C.24 The CSH1 gene encoding Csh 1p was found to be differentially expressed under different growth temperatures.17 At 23˚C to 30˚C temperature, the cell wall of C. albicans contains short, blunt fibrils which allow exposure of hydrophobic proteins on cell surface. Though the hydrophobic proteins are also present at 37˚C, masked hydrophobicity is not measured in the conventional assays of hydrophobicity measurement and the cells are said to be hydrophilic.17,23 Cell surface hydrophobicity varies also with the growth phase of C. albicans. During exponential phase of growth, expression of Csh 1p is higher which decreases in stationary phase.17 Different morphological forms such as yeast cell, pseudohyphae, and true hyphae vary in CSH status. The expression of CSH is reported to play a role in germ tube formation.13,28 Germ tubes of the C. albicans are highly hydrophobic, regardless of the nature of mother yeast cell.29 Hydrophilic cells when grow at 37˚C under germ tube inducing conditions, their hydrophobicity is found to increase prior to germ tube formation.30 Germ tube forms tend to be more hydrophobic than parent yeast cells kept under same environmental condition.18 Whether this increase in CSH is due to enhanced expression of CSH 1p or due to change in the structure of surface fibrillar layer is not clear. CSH expression may change also with the change of growth media. Sabouraud dextrose broth gives less percentage of CSH; defined Lee-Buckley-Campbell medium produces cells with high CSH, while cells grown in yeast nitrogen base (YNB) medium are with comparatively low CSH.12,27 The effect of specific media components that cause actual alterations in CSH have not been investigated systematically.17
Csh as A Virulence Factor in Candida Albicans
Knockout of CSH1 gene showed that the Csh1p is the principal protein affecting CSH.23 An in vivo study in mice has shown that the hydrophobic cells of Candida are more virulent than hydrophilic cells.13,31 Presence of hydrophobic proteins on surface may influence the initial distribution of yeast cells in the body and determine a site for colonization.28 Hydrophobic cells are more rapidly engulfed by polymorphonucleated neutrophils (PMN) than hydrophilic cells. However, switching of these engulfed cells to germ tube avoids the killing by PMN.32 It is reported that hydrophobic cells lack the 1,2-β mannotetrose groups which are recognized by certain macrophages and thereby less susceptible to phagocytosis through mannose-receptor-mediated process.14,15 The organisms displaying surface hydrophobicity are found to be more capable of adapting to stringent nutritional conditions. This may be because increased CSH can act as a biosurfactant which help to dissolve hydrocarbon aggregates or insoluble compounds.20 Expression of CSH may also allow the colonization of C. albicans at a site in the host body which is usually unsuitable for growth.20,30
Role of Csh in Adhesion and Biofilm Formation
Candida albicans and other closely related Candida species are the major agents of hospital acquired infections. Superficial or systemic candidiasis is usually found to be associated with the ability of C. albicans to adhere on biotic or abiotic substrates.33 Adhesion of C. albicans to host tissues or implanted devices may involve specific or non-specific adhesion mechanisms. Specific adhesion mechanism involve the ligand-receptor mediated adhesion such as fibrinogen binding proteins, laminin binding proteins, fibronectin binding proteins, collagen binding proteins, agglutinin like sequence (Als) proteins, mannoproteins, and adhesins.19,34-36 Non-specific or non ligand-receptor mediated adhesion is the most common way of adhesion. It involves physical forces such as, electrostatic interactions, van der waals interactions and hydrophobic interactions.37,38 First phase in adhesion is usually a non-specific and reversible phase which involves some or all of the non-specific interactions mentioned above.39 Surface hydrophobicity or hydrophobic interactions play important role in non-specific adhesion of C. albicans to host tissues or implanted medical devices.40 Hydrophobic cells get excluded from the water or aqueous environment due to interaction of water molecules within itself than that of with hydrophobic or non-polar particles.20 Hydrophobic microorganisms thus tend to remain close to the liquid- solid interface. As a result such hydrophobic cells can easily interact with and adhere on the solid surfaces,41 while hydrophilic cells tend to be dispersed in the aqueous environment. CSH is found to be involved in non-specific (non-ligand-receptor mediated) adherence of Candida cells to host tissues, extracellular matrix proteins and to the implanted medical devices.23,29 Cloning and functional analysis of a C. albicans gene that encodes for 38-kDa Csh 1p suggested that Csh 1p have a role in adhesion to epithelial cells, endothelial cells, fibronectin and other extracellular matrix proteins in the host.23 In many cases, the CSH status of C. albicans is found to correlate positively with the adhesion of C. albicans to host tissues (such as buccal epithelial cells, vaginal epithelial cells and extracellular protein matrix). For example, it is revealed that adhesion of C. albicans to buccal epithelial cells and acrylic denture materials increased with increase in cell surface hydrophobicity.40,42 During adhesion of C. albicans to buccal epithelial cells, the outer fibrillar layer becomes condensed or dispersed so that contact between inner layer of Candida cell wall and epithelial membrane is possible. It indicates that hydrophobic proteins in the matrix of C. albicans cell wall are responsible for adhesion to host tissue.42 Similarly, hydrophobicity correlates with C. albicans adherence to various prosthetic materials and medical devices.38,43 Few reports claim that there is no significant correlation between CSH and adhesion. Instead, various factors may be working together to govern CSH and its involvement in C. albicans adhesion to solid substrates.44 For example, no correlation is observed between hydrophobicity of C. albicans and its adhesion to buccal epithelial cells (BECs).39 Reinhart and coworkers (1988) showed absence of relation between C. albicans CSH and adherence to acrylic surfaces.44 However, it is speculated that these differences in results may be due to different methodologies used for CSH determination.34,40 It is suggested that adhesion of C. albicans to plastic surfaces is influenced by CSH only if the nature of plastic surface is hydrophobic.38,39
Adhesion helps in colonization of Candida and prevents it from being washed away from the site of infection.36 Adhesion of C. albicans to the host tissues or biomaterials usually leads to biofilm formation.5 Studies have clarified that CSH of various strains/ isolates of C. albicans, C. tropicalis as well as C. parapsilosis correlate with their ability to form biofilms.45-47 Interestingly, it has been found that expression of CSH1 gene is up regulated in biofilm cells compared to that of planktonic cells.48 Reports indicate that CSH is an important pathogenicity factor which is involved in adhesion and biofilm formation. Data from US National Nosocomial Infections Surveillance system shows that half of the nosocomial infections caused by C. albicans are associated with adhesion and biofilm formation on host tissues or implanted medical devices.33,35 It is observed that adhered cells behave differently than the planktonic cells of Candida.34 Colonization of medical devices implanted in a patient may lead to device related infections.49 Cells adhered to a solid surface can withstand host immune defense and lead to biofilm formation which may act as a source of reinfections.5 Biofilm growth causes failure of organ system, resistance to antibiotic treatment and severe systemic candidiasis that eventually results in death.50,51
Csh as A Drug Target
Emergence of drug resistant strains and side effects due to toxicity limit the use of most of the available antifungal drugs.9 In addition, there is increase in the incidence of biofilm associated candidiasis. As such, new strategies need to be explored against multiple drug resistant infections of C. albicans.52 Most of the available antibiotics kill the pathogenic microorganisms to cure the infection; however, a small number of mutants which are resistant to the antibiotics may survive and grow. This kind of natural selection is responsible for the emergence of drug resistant population of microorganisms. To avoid this, a novel strategy of prevention of specific virulence attributes instead of killing the pathogen has been proposed.53 Inhibition of CSH may be used to restrict the pathogen (without killing it) so that selection and emergence of drug resistant population of C. albicans can be avoided. Modulation of CSH may interfere with the ability of C. albicans to adapt to various changes in the microenvironment, its response to the host immune system, adhesion to host tissue surfaces, formation of biofilms and thus to establish the infections. Various studies indicate that antifungal drugs exert effect on pathogenicity of Candida through modulation of CSH. For example, in a study with ten isolates each of C. albicans and C. tropicalis from HIV-infected individuals, CSH is evaluated following one hour exposure to sub-therapeutic concentrations of nystatin, amphotericin B, ketoconazole, fluconazole and 5-flurocytosine. The results reveal that exposure to these antifungals reduces the CSH in C. albicans and C. tropicalis isolates to varying degrees from 14 to 34%.54 Ellepola and Samaranayake (1998) have demonstrated that exposure to antimycotics nystatin, 5-fluorocytosine, ketoconazole and fluconazole decreases CSH of oral isolates of C. albicans as well as interfere with adhesion to buccal epithelial cells.55 One of the studies has analyzed the effects of sub-lethal concentrations of polyenes and azoles on C. dubliniensis. It shows that compared with control, exposure to amphotericin B and ketoconazole cause a significant suppression of CSH; whereas, fluconazole is inefficient in suppression of CSH.56 Chlorhexidine gluconate (CG), a widely used antiseptic in routine dental practice due to its broad-spectrum antimicrobial (also anti Candida) activity is demonstrated to suppress CSH in C. albicans isolates. 0.0025% and 0.005% of CG which is much lower than the concentration therapeutically used (i.e. 0.2%) for mouthwash, lower the CSH values by 21% and 45%, respectively. This activity may be through the effect of CG on cell wall structure and cell surface proteins.57
Farnesol which is a quorum sensing molecule in C. albicans lowers down the CSH of C. albicans. More than 70% reduction in CSH of cells treated with 100 µM concentration of farnesol compared to control is reported.45 Interestingly, the sesquiterpene (farnesol) is also a constituent of plant essential oils.58 Plants are rich sources of bioactive molecules with antimicrobial properties.59 Various phytochemicals have been evaluated for their antifungal and anti-Candida potential.60-62 It is observed that some of the natural molecules exhibit modulation of CSH and hence specifically inhibit Candida pathogenicity. For example, hydroxytyrosol (HT) which is an antioxidant in olive oil and leaves cause cell wall damage which results into decrease in C. albicans CSH.63 Tetrandrine (TET), a bis-benzyl isoquinoline alkaloid compound present in several natural plant sources significantly reduces the CSH index from 0.73 to 0.56 at 4 µg/ml concentration; while, 32 µg/ml of it lowers the C. albicans CSH to 0.04.64 Sudjana et al. (2012) have demonstrated that treatment with 0.125% Melaleuca alternifolia (tea tree) essential oil decreases the hydrophobicity of C. albicans isolates. They have proposed that modulation of CSH may be one of the potential mechanisms by which adhesion and biofilm formation could be significantly reduced.65 Brucea javanica and Piper betle extracts exert the CSH reducing effect on seven Candida species including, C. albicans, C. tropicalis, C. glabrata, C. parapsilosis, C. krusei, C. dubliniensis and C. lusitaniae. Concentration dependent reduction in hydrophobicity of C. albicans is observed after treatment with water extract of B. javanica. More than 50% lowering of hydrophobicity is observed in C. albicans cells treated with 1 to 3 mg/ml of these extracts, while 6 mg/ml concentration caused 80 to 90% reduction in CSH.66 Sub-MIC concentrations of cinnamaldehyde, citral, eugenol and geraniol ranging from 25-100 µg/ml, are reported to cause recognizable reduction in C. albicans CSH.67 Ethanolic extract of the roots of a Sri Lankan traditional medicinal plant, Pongamia pinnata, possess hydrophobicity lowering potential against C. albicans, at concentrations ranging from 0.8 to 3.2 mg/ml.68 These studies indicate that plant molecules exhibit significant CSH modulation properties.
Conclusion
Cell surface hydrophobicity is an important virulence attribute in C. albicans which contributes to its successful survival inside the host as well as its ability to establish an infection and cause disease. It modulates important virulence attributes like adhesion and biofilm formation and hence contributes to multiple drug resistance associated with these factors. Such an important pathogenicity determinant would also be an attractive antifungal target. Antifungal drugs available against Candida are showed to modulate CSH and affect its pathogenicity. Interestingly, phytochemicals exhibit potential to lower CSH and thereby prevent adhesion, biofilm formation and overall pathogenicity of C. albicans. Efforts need to be done for development of plant molecules as specific inhibitors of CSH which may prove as a novel strategy to overcome the limitations of available antifungal drugs.
Acknowledgements
SMK acknowledge Prof. P. B. Vidyasagar, Hon’ble Vice-Chancellor of the SRTM University, Nanded, for support and encouragement.
Conflict of Interest
RRG and JSR have equal contribution to this review. The manuscript has no conflict of interest.
Funding Source
The manuscript has not received any financial support or funding.
References
- Sardi J.C,Scorzoni O.L, Bernardi T, Fusco-Almeida A.M, Giannini M.M. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013;62:10–24.
CrossRef - Raut J.S, Doke S.K, Karuppayil,S.M. Yeast biofilm in the context of human health and disease. In: Yeast diversity in human welfare (Satyanarayana T, Kunze G, editors). Singapore: Springer Nature. 2017;137–162.
CrossRef - Kim J, Sudbery P. Candida albicans, a major human fungal pathogen. Microbiol. 2011;49:171–177.
CrossRef - Mishra N.N, Prasad T, Sharma N, Payasi A, Prasad R, Gupta D.K, Singh R. Pathogenicity and drug resistance in Candida albicans and other yeast species. Acta Microbiol. Immunol. Hungar. 2007;54:201.
CrossRef - Shinde R.B, Raut J.S, Karuppayil S.M. Biofilm formation by Candida albicans on various prosthetic materials and its fluconazole sensitivity: A kinetic study. Mycoscience. 2012;53:220.
CrossRef - Raut J.S, Chauhan N.M, Shinde R.B, Karuppayil S.M. Inhibition of planktonic and biofilm growth of Candida albicans reveals novel antifungal activity of caffeine. Med. Plants Res. 2013;7:777-782.
- Molero G, Diez-Orejas R, Navarro-Garcia F, Monteoliva L, Pla J, Gil C, Sanchez-Perez M, Nombela C. Candida albicans: genetics, dimorphism and pathogenicity. Microbiol. 1998;1:95–106.
- Miller L.G, Hajjeh R.A, Edwards J.E. Estimating the cost of nosocomial candidemia in the United States. Infect. Dis. 2001;32:1110.
CrossRef - Andes D, Nett J, Oschel P, Albert R, Marchillo K, Pitula A. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. National Nosocomial Infections Surveillance system. American J. Med. 2004;91:86–89.
- Odds F.C. Candida species and virulence. Virulence. 1994;50:313.
- Sprague G.F, Winans S.C. Eukaryotes learn how to count: Quorum sensing by yeast. Genes Develop. 2006;20:1045.
CrossRef - Hazen K.C. Cell surface hydrophobicity of medically important fungi, especially Candida In: Microbial cell surface hydrophobicity (Doyle RJ, Rosenberg M, editors). Washington, DC: ASM Press. 1990;249–295.
- Antley P.P, Hazen K.C. Role of yeast cell growth temperature on Candida albicans virulence in mice. Immun. 1988;56:2884–2890.
- Masuoka J, Hazen K.C. Cell wall protein mannosylation determines Candida albicans cell surface hydrophobicity. Microbiology. 1997;143:3015.
CrossRef - Masuoka J. Surface glycans of Candida albicans and other pathogenic fungi: Physiological roles, clinical uses, and experimental challenges. Microbiol. Rev. 2004;17:281–310.
CrossRef - Masuoka J, Hazen K.C. Differences in the acid-labile component of Candida albicans mannan from hydrophobic and hydrophilic yeast cells. Glycobiology. 1999;11:1281.
CrossRef - Singleton D.R, Hazen K.C. Differential surface localization and temperature dependent expression of the Candida albicans CSH1 protein. Microbiology. 2004;150:285.
CrossRef - Hazen K.C, Hazen B.W. Hydrophobic surface protein masking by the opportunistic fungal pathogen Candida albicans. Immun. 1992;60:1499–1508.
- Chaffin W.L, Lopez-Ribot J.L, Casanova M, Gozalbo D, Martinez J.P. Cell wall and secreted proteins of Candida albicans: Identification, function, and expression. Mol. Biol. Rev. 1998;62:130–180.
- Hazen K.C, Lay J.G, Hazen B.W, Fu R.C, Murthy S. Partial biochemical characterization of cell surface hydrophobicity of Candida albicans. Immun. 1990;58:3469–3476.
- Casanova M, Lopez-Ribot J.L, Monteagudo C, Llombart-Bosch A, Sentandreu R, Martinez J.P. Identification of a 58 kilodalton cell surface fibrinogen- binding mannoprotein from Candida albicans. Immun. 1992;60:4221–4229.
- Glee P.M, Sundstrom P, Hazen K.C. Expression of surface hydrophobic proteins by Candida albicans in vivo. Immun. 1995;63:1373–1379.
- Singleton D.R, Masuoka J, Hazen K.C. Cloning and analysis of a Candida albicans gene that affects cell surface hydrophobicity. Bacteriol. 2001;183:3582–3588.
CrossRef - Hazen K.C, Wu J.G, Masuoka J. Comparison of the hydrophobic properties of Candida albicans and Candida dubliniensis. Immun. 2001;69:779–786.
CrossRef - Singleton D.R, Masuoka J, Hazen K.C. Surface hydrophobicity changes of two Candida albicans serotype Bmnn4Δ mutants. Cell. 2005;4:639–648.
- Masuoka J, Hazen K.C. Cell wall mannan and cell surface hydrophobicity in Candida albicans serotype A and B strains. Immun. 2004;72:6230–6236.
CrossRef - Hazen K.C, Plotkin B.J, Klimas D.M. Influence of growth conditions on cell surface hydrophobicity of Candida albicans and Candida glabrata. Immun. 1986;54:269–271.
- Rodrigues A.G, Mardh P.A, Pina-Vaz C, Martinez-de-Oliveira J, Fonseca A.F. Germ tube formation changes surface hydrophobicity of Candida Inf. Dis. Obstetric and Gynecol. 1999;7:222–226.
- Lopez-Ribot J.L, Casanova M, Martinez J.P, Sentandreu R. Characterization of cell wall proteins of yeast and hydrophobic mycelial cells of Candida albicans. Immun. 1991;59:2324–2332.
- Hazen B.W, Hazen K.C. Dynamic expression of cell surface hydrophobicity during initial yeast cell growth and before germ tube formation of Candida albicans. Immun. 1988;56:2521–2525.
- Hazen K.C. Relationship between expression of cell surface hydrophobicity protein1 (csh1p) and surface hydrophobicity properties of Candida dubliniensis. Curr Microbiol. 2004;48:447.
CrossRef - Torosantucci A, Romagnoli G, Chiani P, Stringaro A, Crateri P, Mariotti, S, Teloni R, Arancia G, Cassone A, Nisini R. Candida albicans yeast and germ tube forms interfere differently with human monocyte differentiation into dendriatic cells: a novel dimorphism-dependent mechanism to escape the host immune response. Immun. 2004;72:833–843.
- Douglas L.J. Medical importance of biofilms in Candida Rev. lberoam. Micol. 2002;19:139–143.
- Ener B, Douglas L.J. Correlation between cell surface hydrophobicity of Candida albicans and adhesion to buccal epithelial cells. FEMS Microbiol. 1992;99:37.
CrossRef - Verstrepen K.J, Reynolds T.B, Fink G.R. Origins of variation in the fungal cell surface. Nature Rev. Microbiol. 2004;2:533.
CrossRef - Verstrepen K.J, Klis F.M. Flocculation, adhesion and biofilm formation in yeasts. Microbiol. 2006;60:5–15.
CrossRef - Hobden C, Teevan C, Jones L, O’Shea. P. Hydrophobic properties of the cell surface of Candida albicans: a role in aggregation. Microbiology. 1995;141:1875.
CrossRef - Klotz S.A. Role of hydrophobic interactions in microbial adhesion to plastics used in medical devices. In: Microbial cell surface hydrophobicity (Doyle RJ, Rosenberg M, editors). Washington, DC: ASM Press. 1990;107.
- Kennedy M.J, Rogers A.L, Yancey R.J. Environmental alteration and phenotypic regulation of Candida albicans adhesion to plastic. Immun. 1989;57:3876–3881.
- Panagoda G.J, Ellepola A.N.B, Samaranayake L.P. Adhesion of Candida parapsilosis to epithelial and acrylic surfaces correlates with cell surface hydrophobicity. Mycoses. 2001;44:29–
CrossRef - Klotz S.A. Surface-active properties of Candida albicans. Environ. Microbiol. 1989;55:2119–2122.
- Jabra-Rizk M.A, Falkler W.A, Merz W.G.J, Baqui A.A.M.A, Kelley J.I, Meiller T.F. Cell surface hydrophobicity- associated adherence of Candida dubliniensis to human buccal epithelial cells. lberoam. Micol. 2001;18:17–22.
- Henriques M, Azeredo J, Oliveira R. Adhesion of Candida albicans and Candida dubliniensis to acrylic and hydroxyapatite. Colloids Surf. B: Biointerfaces. 2004;33:235.
CrossRef - Raut J, Rathod V, Karuppayil S.M. Cell surface hydrophobicity and adhesion: a study on fifty clinical isolates of Candida albicans. J. Med. Mycol. 2010;51:131–136.
CrossRef - Cao Y.Y, Cao Y.B, Xu Z, Kang Y, Yao L, Yi X, Zhu Z.Y, Chen W.S, Jiang Y.Y. cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol. Agents Chemother. 2005;49:584–589 .
CrossRef - Borghi E, Sciota R, Biassoni C, Cirasola D, Cappelletti L, Vizzini L, Boracchi P, Morace G. Cell surface hydrophobicity: a predictor of biofilm production in Candida isolates? J. Med. Microbiol. 2011;60:689–690.
CrossRef - Galan‐Ladero M.A, Blanco‐Blanco M.T, Hurtado C, Perez‐Giraldo C, Blanco M.T, Gomez‐Garcia A.C. Determination of biofilm production by Candida tropicalis isolated from hospitalized patients and its relation to cellular surface hydrophobicity, plastic adherence and filamentation ability. Yeast. 2013;30:331.
CrossRef - Bujdakova H, Didiasova M, Drahovska H, Cernakova L. Role of cell surface hydrophobicity in Candida albicansCentral Europ. J. Biol. 2013;8:259–262.
- Kojic E.M, Darouiche R.O. Candida infections of medical devices. Microbiol. 2004;17:255–267.
CrossRef - Ganguly S, Mitchell A.P, Mucosal biofilms of Candida albicans. Opin. Microbiol. 2011;14:380–385.
CrossRef - Cuellar-Cruz M, Vega-Gonzalez A, Mendoza-Novelo B, Lopez-Romero E, Ruiz-Baca E, Quintanar-Escorza M.A, Villagómez-Castro J.C. The effect of biomaterials and antifungals on biofilm formation by Candida species: a review. J. Clin. Microbiol. Inf. Dis. 2012;31:2513–2527.
CrossRef - Nett J.E. Future directions for anti-biofilm therapeutics targeting Candida. Expert Rev. Anti Infect. Ther. 2014;12:375.
CrossRef - Clatworthy A.E, Pierson E, Hung D.T. Targeting virulence: a new paradigm for antimicrobial therapy. Nature Chem. Biol. 2007;3:541.
CrossRef - Anil S, Ellepola A.N.B, Samaranayake L.P. The impact of polyene, azole, and DNA analogue antimycotics on the cell surface hydrophobicity of Candida albicans and Candida tropicalis in HIV infection. Mycopathologia. 2001;153:179.
CrossRef - Ellepola A.N, Samaranayake L.P. The effect of limited exposure to antimycotics on the relative cell-surface hydrophobicity and the adhesion of oral Candida albicans to buccal epithelial cells. Arch. Oral Biol. 1998;43:879–887.
CrossRef - Ellepola A.N, Joseph B.K, Khan Z.U. Changes in germ tube formation and cell‐surface hydrophobicity of oral Candida dubliniensis isolates following brief exposure to sub‐cidal concentrations of polyene and azole antifungal agents. Mycoses. 2014;57:56.
CrossRef - Ellepola A.N, Joseph B.K,Khan Z.U. Changes in the cell surface hydrophobicity of oral Candida albicans from smokers, diabetics, asthmatics, and healthy individuals following limited exposure to chlorhexidine gluconate. Prin. Pract. 2013;22:250–254.
CrossRef - Mo H, Elson E.C. Studies of the isoprenoid mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Biol. Med. 2004;229:567–585.
CrossRef - Raut J.S, Karuppayil S.M. Bioprospecting of plant essential oils for medicinal uses. In: Environment and sustainable development (Fulekar M.H, Pathak B, Kale R.K, editors). India: Springer. 2014;59.
- Sadri A, Khodavandi A, Alizadeh F. Allium sativum, Allium hirtifolium and Allium cepa: The probable quorum-sensing quenching compounds against Candida albicans. Biosci. Biotech. Res. Asia. 2016;13:1457.
- Raut J.S, Karuppayil S.M. Phytochemicals as Inhibitors of Candida Curr. Pharma. Des. 2016;22:4111–4134.
- Raut J.S, Bansode B.S, Jadhav A.K, Karuppayil S.M. Activity of allyl isothiocyanate and its synergy with fluconazole against Candida albicansJ. Microbiol. Biotechnol. 2017;27:685–693.
CrossRef - Zoric N, Horvat I, Kopjar N, Vucemilovic A, Kremer D, Tomic S, Kosalec I. Hydroxytyrosol expresses antifungal activity in vitro. Drug Targets. 2013;14:992–998.
CrossRef - Zhao L.X, Li D.D, Hu D.D, Hu G.H, Yan L, Wang Y, Jiang Y.Y. Effect of tetrandrine against Candida albicans. PloS One. 2013;8:e79671.
- Sudjana A.N, Carson C.F, Carson K.C, Riley T.V, Hammer K.A. Candida albicans adhesion to human epithelial cells and polystyrene and formation of biofilm is reduced by sub-inhibitory Melaleuca alternifolia (tea tree) essential oil. Mycol. 2012;50:863–870.
- Nordin M.A,Wan F, Harun W.H.A, Abdul R.F. An in vitro study on the anti-adherence effect of Brucea javanica and Piper betle extracts towards oral Candida. Oral Biol. 2013;58:1335–1342.
CrossRef - Khan M.S, Ahmad A.I. In vitro influence of certain essential oils on germ tube formation, cell surface hydrophobicity, and production of proteinase and hemolysin in Candida albicans. Natural Pharmaceut. 2012;3:110.
CrossRef - Kanatiwela D.K, Abayasekara C.L, Adikaram N.K, Parahitiyawa N.B, Senanayake D.M, Panagoda G.J. Anti-candidal activity and effect on relative cell surface hydrophobicity of Pongamia pinnata. Afr. J. Microbiol. Res. 2013;7:2948–2956.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





