Manuscript accepted on : 04 April 2017
Published online on: --
Plagiarism Check: Yes
Nithin Vijayakumar and Sangilimuthu Alagar
Department of Biotechnology, Karpagam University, KAHE, Coimbatore, Tamil Nadu-641021.
Corresponding Author E-mail: sangilimuthu.a@kahedu.edu.in
DOI : http://dx.doi.org/10.13005/bbra/2473
ABSTRACT: In the present study, laboratory trail was carried out to determine the effect of partially purified chitinase from Trichoderma viride on certain digestive enzymes of Corcyra cephalonica and its antimicrobial susceptibility against certain microbes. The most dreadful stored food grain pest Corcyra cephalonica (Stainton) was fed with chitinase from Trichoderma viride impregnated flour disk of different concentrations (62.5, 125, 250, 500 and 1000 ppm). After the treatment of five days, gut region was dissected and the inhibitory activity of chitinase against digestive enzymes (amylase, invertase and lactase) was checked. The incorporation of chitinase to the feed induced the reduced level of enzymes such as amylase, invertase and lactase with increasing concentration of chitinase. The decrease in the digestive enzymes such as amylase (75%), invertase and lactase substantiate that the chitinase can induce changes in the insect physiology to a great extent. Based on the results observed, the chitinase from Trichoderma viride can be promoted as a biocontrol agent against the stored food grain pests. Chitinase showed appreciable antibacterial activity against the gram positive bacteria such as Staphylococcus aureus, Streptococcus mutans; gram negative bacteria Klebsiella pneumonia, Escherichia coli, Pseudomonas aeruginosa and antifungal activity against Aspergillus niger and Candida albicans.
KEYWORDS: Amylase; invertase; lactase; Corcyra cephalonica; integrated pest management; stored grain pest
Download this article as:| Copy the following to cite this article: Vijayakumar N, Alagar S. Consequence of Chitinase from Trichoderma Viride Integrated feed on Digestive Enzymes in Corcyra Cephalonica (Stainton) and Antimicrobial Potential. Biosci Biotech Res Asia 2017;14(2). |
| Copy the following to cite this URL: Vijayakumar N, Alagar S. Consequence of Chitinase from Trichoderma Viride Integrated feed on Digestive Enzymes in Corcyra Cephalonica (Stainton) and Antimicrobial Potential. Biosci Biotech Res Asia 2017;14(2). Available from: https://www.biotech-asia.org/?p=23980 |
Introduction
In developing countries like India, to meet the increased crop yield and to deliver the frequently aggregating food demand, chemical pesticides are mostly used. In India, rice has shaped the society, diets and economy of thousands of millions of common people.1 Except India, many developing countries are suffering from unceasing food shortage that are originated by factors such as the repeatedly low productivity and as post harvest lost due to insect pest.2 A number of reports, put forwarded that, the post harvest losses exceeding 20% is generally caused by the infestation of storage grain pests such as Corcyra cephalonica (Lepidoptera), Ephestia cautella (Lepidoptera) Rhyzopertha dominica (Coleoptera), Sitophilus granaries (Coleoptera), Tribolium castaneum (Coleoptera) and Trogoderma granarium (Coleoptera). At the present time, storage insect pest management strategies were pointed in the direction of synthetic pesticide use.3 Synthetic pesticides, for example methyl bromide and phosphine fumigants are considered as the most effectual treatments to manage stored pests for decades. Due to the continual usage of these synthetic pesticides directs to develop resistance and the greater parts of them have been forbidden. In developed countries, certain synthetic pesticides are banned due to high levels of toxicity to mankind, ozone depletion and non biodegradable properties.4,5 The need of effectual pest management approaches has improved to challenge the risk to food security caused by the infestation of these stored food pests.6,7
Digestion is a point of insect physiology on which a few research has been done until now. Amylase (hydrolyzes starch to maltose), maltase (hydrolyzes maltose to glucose), invertase (hydrolyzes sucrose to fructose and glucose), lactase (hydrolyzes lactose to form glucose and galactose), lipase (degrade fats into fatty acids and glycerol), pepsin (in acidic condition breaks proteins into peptones), trypsin (in a alkaline condition it breaks down complex proteins into peptones) and finally erpsin (splits peptones into amino-acids) are the major digestive enzymes present in every insect larvae.8,9 These digestive enzymes are seen in salivary glands, foregut, midgut and gastric oeca. An artificial diet induces metabolic defects in treated larvae of lepidopteran insects, poor feeding, reducing weight gain and morphological changes.10,1 This may occur due to the adverse activity of these digestive enzymes to digest and utilize the ingested food.
The rice moth, Corcyra cephalonica (Stainton), is a widespread common cosmopolitan stored food grain pest in most of the tropical areas.11 It causes huge losses, both quantitative and qualitative damage of stored food grains, dried food stuffs and their products.12 In general, the grains and cereals are having rich carbohydrate and protein content. Usually, carbohydrates and proteins are very efficiently utilized by insects. The utilization of these nutrients depends on the digestive enzymes present in the gut region.13
Subsequently, this study is to explore the effects of partially purified chitinase from Trichoderma viride as an inhibitory agent on the digestive enzymes (amylase, invertase and lactase) of Corcyra cephalonica (Stainton) and as an antimicrobial agent against selected microbes. Thereby, we have to elucidate whether this chitinase can be used as a biocontrol agent against the stored food pest C. cephalonica.
Material and Methods
Production of Crude Chitinase Enzyme
Production and purification of chitinase from T. viride were carried out according to Vijayakumar et al., (2017)14. The fungal isolate of T. viride was grown in the potato dextrose broth (PDB) containing the chitinase production enhancing micro and macro nutrients along with colloidal chitin as a substrate. These were cultured in an Erlenmeyer flask (250 mL) and kept for incubation (eight days) in a shaker. The cultured broth was centrifuged (12, 000 rpm; 10 min; 4°C). 10 mL of supernatant was taken and added with equal volume of wet colloidal chitin (10 %) in 0.2 M phosphate buffer (pH 6.5). The suspension thus obtained was hold in a boiling water bath (50°C) for 1 hr. After the incubation, NaOH (1 %) was added, mixed vigorously and centrifuged (6000 rpm; 10 min; 4°C). The supernatant thus obtained was collected and it was allowed for ammonium sulphate precipitation, allowed to stand overnight. After the overnight incubation, the obtained precipitate was centrifuged (10, 000 rpm; 20 min; 4°C). The precipitate obtained was suspended in small amount of 20 mM citrate phosphate buffer (pH 7.8) and dialyzed against the same buffer. The obtained partially purified chitinase was used for the further studies.
Maintenance of Corcyra Cephalonica
The colony of C. cephalonica (Lepidoptera: Pyralidae) was maintained under standard conditions at 28±1ºC, ≤ 65 % relative humidity, 12:12 light-to-dark photoperiod and maintained on food media contained pearl millet grains, groundnut kernals, sulphur and antibiotic agents.11,1
Preparation of Chitinase Incorporated flour Disks
The chitinase impregnated flour disks for C. cephalonica was prepared based on the diet earlier designed by Vijayakumar et al. (2016)1. Basic ingredients used in diets were pearl millet flour and partially purified chitinase from T. viride along with Milli Q water in five different concentrations (62.5, 125, 250, 500 and 1000 ppm). The above mentioned suspension was aliquoted (250 µL) and placed on a polystyrene petri dish to form disks and left in laminar air flow chamber overnight to air dry. The disks were then moved to an incubator to equilibrate at 30±1ºC for 48 hr. After the incubation, the IV instar C. cephalonica larvae were fed for five days and checked for the digestive enzymes (amylase, invertase and lactase).
Evaluation of Digestive Enzymes in Gut Region
For evaluating the effect of chitinase from T. viride on the gut digestive enzymes generally amylase, invertase and lactase were studied by determination of enzyme activity in the homogenized midgut samples collected from the treated IV instar larvae of C. cephalonica. Twenty five larvae from the control (treated with flour disk with Milli Q water served as control) and chitinase treated category were selected in this study.
Under aseptic condition, the midgut portion of the respective category was dissected out in ice cold saline and dissected midgut was suspended in saline solution followed by successive washing in sterile distilled water to remove undigested food waste and fat bodies. Washed portion was homogenized in ice cold citrate phosphate buffer (pH 7.6) using micropestle (Eppendorf). Homogenate thus obtained was centrifuged (8500 rpm; 15 min; 4ºC) and the collected supernatant was used as the enzyme source.
Di-nitrosalycilic acid (DNS) method was employed to check the activity of different digestive enzymes from the partially purified chitinase treated midgut of C. cephalonica IV instar larvae. For the determination of amylase inhibition activity15 of chitinase, the above mentioned enzyme solution was mixed with the buffer (5 mM calcium chloride; 50 mM Sodium acetate and 10 mM Sodium chloride). The substrate used for amylase inhibition assay is 1 % starch (w/v). The above mentioned enzyme mixture was incubated for 20 min at room temperature (RT: 37±2ºC). After incubation, 96 mM DNS (equal volume) was added and kept it in boiling water bath (15 min, 50ºC) to stop the reaction and the absorbance was read at 535 nm using Ultra-violet Visible spectrophotometer (UV-Vis Spectrophotometer) (Agilent Cary 60). The amylase inhibition was calculated as follows.
![]()
Evaluation of activity of invertase and lactase in gut region of chitinase treated IV instar C. cephalonica larvae were also done by DNS method. The substrate used for the determination of invertase activity was sucrose in 300 mM acetate buffer (pH 6.9) and for lactase was lactose in 56 mM acetate buffer (pH 6.9). The activity was calculated form a standard curve using known concentration of glucose.
Microorganisms for Antimicrobial Susceptibility
Gram positive strains [Staphylococcus aureus (MTCC No. 2940), Streptococcus mutans (MTCC No. 497)], gram negative strains [Klebsiella pneumoniae (MTCC No. 4031), Escherichia coli (MTCC No. 1195), Pseudomonas aeruginosa (MTCC No. 4673)] bacterium and fungal cultures Aspergillus niger (MTCC No. 4325) and Candida albicans (MTCC No. 3017) were chosen for checking the antimicrobial activity of chitinase from T. viride.
Antimicrobial Susceptibility
Antimicrobial susceptibility of partially purified chitinase from T. viride against certain gram positive, gram negative bacterium and fungal strains were determined by agar well diffusion method. Bacterial cells were inoculated in Luria Britanicia Broth (LB Broth) and fungal organisms in Potato dextrose broth (PDB) to obtain a cell density of 108 CFU/mL (0.5 McFarland standard). The 20 µL culture was swabbed on to Mulller Hinton Agar (MHA) for bacterial strains and Potato Dextrose Agar (PDA) plates for fungal strains and wells were cut using a cork borer. Different concentrations (25, 50 and 100 µL) of chitinase (1 mg.mL-1) were added in each well. Streptomycin (10 µL from 10 mg.mL-1 stock) served as a positive control for bacteria and Clotrimazole (10 µL from 10 mg.mL-1 stock) for fungal strains. Plates were incubated at 37ºC for 24 to 48 hr. The zone of inhibition produced by the partially purified chitinase from T. viride was measured in millimetre.
Statistical Analysis
Data from amylase, invertase and lactase were expressed as the mean of three replicates. The inhibitory concentration (IC50) was calculated using ED50PlusV1.0.
Results and Discussion
Effect of Chitinase Towards Gut Enzymes of C. Cephalonica
The current results evidently point towards that all the tested enzyme activities were abridged in the treatment of the C. cephalonica with diverse concentrations of partly purified chitinase according to a dose-dependent mode. Partially purified chitinase was tested for the gut α-amylase inhibitory activity of C. cephalonica. At a minor concentration of 62.5 ppm of chitinase, there was 47 % inhibition of the α-amylase activity. The inhibition of the α-amylase activity was found to be in a dose-dependent style. At the concentration of 1000 ppm, almost absolute inhibition of amylase activity (76 %) was conquered (Figure 1A). The IC50 value for amylase inhibition was calculated as 70.966 ppm. Invertase and lactase activities were reduced at maximum level according to a dose-dependent mode (Figure 1B & C). Chitinase treatment also revealed distinct reduction of the extracellular gut enzyme production potential. The IC50 for invertase and lactase was calculated as 965.79 and 1065.34 ppm respectively.
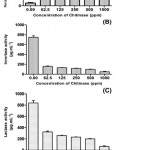 |
Figure 1: Effect of partially purified chitinase from Trichoderma Viride on gut enzyme profile of Corcyra cephalonica (Stainton) A) Alpha amylase inhibition activity B) Invertase Activity C) Lactase Activity
|
In this study inhibitory activity of partially purified chitinase from T. viride against the digestive enzymes such as amylases, invertase and lactase were examined. The enzymatic analysis using an enzymatic approach showed a dose-dependent inhibition. Here in our study we observed the reduction in gut enzyme activity. It gives unambiguous substantiation for the inhibitory activity of chitinase from T. viride against the amylase, invertase and lactase enzymes. Insect physiology is profoundly dependent on digestive enzymes which convert complex molecules into simple components which are obligatory for the proper growth and metabolism.16,13 A range of enzymes (amylase, invertase and lactase) activity of the midgut revealed for exogenous proteins, lipids, sugars and even in the ATP synthesis. The results of this study undoubtedly point out that there is substantial drop in all the tested digestive enzymes (midgut) activity due to the abridged food substrate exploitation by chitinase incorporation. We all are aware that C. cephalonica is always infested in the stored food grains which hold a hefty amount of starch and carbohydrate. The starch which is consumed by the insect has to be transformed into the absorbable form, maltose.8 Here the amylase inhibition is going to a higher range in consent with the concentration of the chitinase. In the same way invertase and lactase is converting the higher molecules into subunits such as sucrose, fructose, glucose and galactose correspondingly.
We already reported the effect of chitinase incorporated feed on C. cephalonica and its nutritional indices. It shows a 99% of feeding deterrence index at a concentration of 500 ppm chitinase incorporated feed1. It is also reported that due to the incorporation of chitinase in the feed leads to poor feeding and thereby leads to the decrease in growth rate.
Antimicrobial Susceptibility
Agar well diffusion method was employed for the evaluation of antimicrobial activity of the partially purified chitinase from T. viride. The chitinase possessed appreciable anti-microbial activity against the selected pathogens. Chitinase showed excellent activity for S. aureus followed by K. pneumoniae and S. mutans (Figure 2A, B, C, D & E) and comparable activity towards A. niger (Figure 4A & B). The zone of inhibition exhibited by these microbes at different concentrations is displayed (Figure 3 & 5). For both the fungal species chosen for the antifungal susceptibility, partially purified chitinase from T. viride showed almost similar activity checked in the three different concentrations. The zone of inhibition widths are in the range of 20-24 mm for S. aureus, 14-18 mm for K. pneumoniae, 15-18 mm for P. aeruginosa, 13-17 mm for S. mutans and 14-15 mm for E. coli. In the case of fungal strains checked, the zone of inhibition widths are in the range of 14-19 mm for A. niger and 14-18 mm for C. albicans. Increasing concentration of the chitinase from T. viride inhibits the growth of gram positive and gram negative organisms to a great extend. Similar results are obtained in the case of the screened fungal strains. These results strongly support that the chitinase has strong antimicrobial activity.
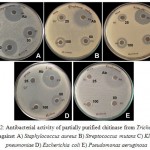 |
Figure 2: Antibacterial activity of partially purified chitinase from Trichoderma viride against A) Staphylococcus aureus B) Streptococcus mutans C) Klebsiella pneumoniae D) Escherichia coli E) Pseudomonas aeruginosa
|
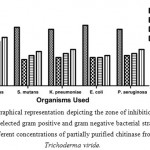 |
Figure 3: Graphical representation depicting the zone of inhibition formed against selected gram positive and gram negative bacterial strains by different concentrations of partially purified chitinase from Trichoderma viride.
|
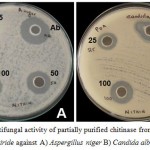 |
Figure 4: Antifungal activity of partially purified chitinase from Trichoderma viride against A) Aspergillus niger B) Candida albicans
|
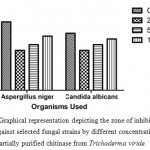 |
Figure 5: Graphical representation depicting the zone of inhibition formed against selected fungal strains by different concentrations of partially purified chitinase from Trichoderma viride
|
Bacterial and fungal infections are gradually raising worldwide dilemma in the stored food grains due to the augment in moisture content within the granaries and godowns. This moisture content paves a way to the friendliness environment for the development of several pathogens to the stored food grains. In our study it intended in co-operation with the control of microbes and pests that can affect the stored food materials. Antimicrobials, in general can be classified into two categories, according to their chemical composition. One is organic based and the other one is inorganic based materials. Inorganic based antimicrobial agents’ show signs of long shelf life and is resistant to high temperature17, 18. Nevertheless, on the other hand they are having feeble action and a large dosage has to be employed for the elimination of these microbes. In case of organic antimicrobial agents, they are proficient of signifying a broad range competence and outstanding inhibition activity towards microbes in contrast to inorganic antimicrobial agents.19 Quite a lot of studies on the antimicrobial properties of natural products like enzymes and microbial peptides were reported in the literature. In this study we have confirmed the antimicrobial effectiveness of partially purified chitinase from T. viride by agar well diffusion method. The inhibition zone diameters were obtained for chitinase.
Conclusion
Results showed an enhanced amylase inhibitory activity and reduce the invertase and lactase activity, which leads to the poor feeding and thereby a reduced growth rate in C. cephalonica. The results betoken that chitinase can be used as an appropriate biocontrol agent against the most dreadful stored grain pest C. cephalonica. In addition, chitinase from T. viride showed enhanced antimicrobial activity against the S. aureus, S. mutans, K. pneumonia, E. coli, P. aeruginosa, A. niger and C. albicans.
Acknowledgement
We are thankful to Karpagam University, Coimbatore, India for providing laboratory infrastructural facilities.
References
- Vijayakumar N., Alagar S., Madanagopal N. Effects of chitinase from Trichoderma viride on feeding, growth and biochemical parameters of the rice moth, Corcyra cephalonica J. Entomol. Zool. Stud. 2016;4(4):520-23.
- Waongo A., Ba N. M., Dabiré-Binso L. C., Sanon A. Diversity and community structure of insect pests developing in stored sorghum in the Northern-Sudan ecological zone of Burkina Faso. Stored Prod. Res. 2015;63:6–14.
CrossRef - Chigoverah A. A., Mvumi B. M. Efficacy of metal silos and hermetic bags against stored-maize insect pests under simulated smallholder farmer conditions. Stored Prod. Res. 2016;69:179–89.
CrossRef - Fields P. G., White N. D. G. Alternatives to methyl bromide treatments for stored- products insects and quarantine insects. Rev. Entomol. 2002;47:331-59.
CrossRef - Mansour E. E., Mi F., Zhang G., Jiugao X., Wang Y., Kargbo A. Effect of allyliso thiocyanate on Sitophilus oryzae, Tribolium confusum and Plodia interpunctella toxicity and effect on insect mitochondria. Crop Prot. 2012;33:40-51.
CrossRef - Nyagwaya L. D., Mvumi B. M., Saunyama I. G. Occurrence and distribution of Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) in Zimbabwe. J. Trop. Insect Sci. 2010;30:221-31.
CrossRef - Muatinte B. I., Van den Berg J., Santos L. A. Prostephanus truncatus in Africa: a review of biological trends and perspectives on future pest management strategies. Crop Sci. J. 2014;22:237-56.
- Swingle H. S. Digestive enzymes of an insect. Ohio J. Sci. 1925;25(5):209-18.
- Vijayan S., Imani J., Tanneeru K., Guruprasad L., Kogel K. H., Kirti P. B. Enhanced antifungal and insect alpha amylase inhibitory activities of Alpha-TvD1, a peptide variant of Tephrosia villosa defensin (TvD1) gene through in vitro Peptides. 2012;33:220-29.
CrossRef - Hasaballa Z. A., Abdel-Baki S. M., Darwish Y. A. Type of food and gamma irradiation as factors affecting the larval growth of Ephestia kuehniella (Zell.), Corcyra cephalonica (Staint.) and Plodia interpunctella (Hubn.). Assiut J. Agric. Sci. 1990;12:215–23.
- Allotey J., Azalekor W. Some aspects of the biology and control using botanicals of the rice moth, Corcyra cephalonica (Stainton) on some pulses. Stored Prod. Res. 2000;36(3):235–43.
CrossRef - Rajendran S. Post harvest pest losses. In pimental D. (Ed). Encyclopedia of pest management., New York: Marcker Dekker. Inc. 2002;654-56.
- Boshra S. A. Effect of gamma irradiation on food consumption, assimilation and digestive enzymes in Ephestia cautella (Walker) larvae. Stored Prod. Res. 2007;43(1):49–52.
CrossRef - Vijayakumar N., Alagar S., Madanagopal N. Biocontrol potential of fungal chitinase from higher yielding Trichoderma viride against Corcyra cephalonica (Stainton). J. Pharm. Bio. Sci. 2017;8(2):(B)447-52.
- Bernfeld P., Amylases A and B. Methods in enzymology, New York: Academic Press, Inc. 1995;149–58.
- Bharani R. S. A., Namasivayam S. K. R. Biogenic silver nanoparticles mediated stress on developmental period and gut physiology of major lepidopteran pest Spodoptera litura (Fab.) (Lepidoptera: Noctuidae)—An eco-friendly approach of insect pest control. Environ. Chem. Eng. 2017;5(1):453–67.
CrossRef - Tong G., Yulong M., Peng G., Zirong X. Antibacterial effects of the Cu(II)-exchanged montmorillonite on Escherichia coli K88 and Salmonella choleraesuis. Microbiol. 2005;105:113–22.
CrossRef - Wang X., Du Y., Luo J., Yang J., Wang W., Kennedy J. F. A novel biopolymer rectorite nano composite with antimicrobial activity. Polym. 2009;77:449–56.
- He H. D., Yang P., Yuan W., Shen R. L. Frost. A novel organo-clay with antibacterial activity prepared from montmorillonite and Chlorhexidiniacetas. Colloid Interface Sci. 2006;297:235–43.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





