Manuscript accepted on : 02 March 2017
Published online on: --
Plagiarism Check: Yes
The Effect of Adding Different Concentrations of Cows’ Milk on the Antioxidant Properties of Coffee
Ayat B. Al-Ghafari, Rahaf H. Alharbi, Manal M. Al-Jehani, Shoroq A. Bujeir, Huda A. Al-Doghaither and Ulfat M. Omar
Department of Biochemistry, Faculty of Science, King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia.
Corresponding Author E-mail: abalghafari@kau.edu.sa
DOI : http://dx.doi.org/10.13005/bbra/2433
ABSTRACT: Polyphenols have been widely studied and considered as a health promoting and disease preventive agents in humans. Several studies investigated the antioxidant properties of polyphenols and their abilities to eliminate free radicals. In this study, the antioxidant activity of coffee in the presence of different types of cows’ milk at different concentrations, 10% or 20%, was investigated. Our results showed that milk could either enhance the scavenging of DPPH or decrease the metal chelating and metal reducing activity of polyphenol. However, more investigations are required to evaluate the mechanisms by which fats in milk can alter the antioxidant activity of coffee.
KEYWORDS: metal chelating reducing power; semi-skimmed milk; skimmed milk; scavenging activity;Whole milk;
Download this article as:| Copy the following to cite this article: Al-Ghafari A. B, Alharbi R. H, Al-Jehani M. M, Bujeir S. A, Al-Doghaither H. A, Omar U. M. The Effect of Adding Different Concentrations of Cows’ Milk on the Antioxidant Properties of Coffee. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Al-Ghafari A. B, Alharbi R. H, Al-Jehani M. M, Bujeir S. A, Al-Doghaither H. A, Omar U. M. The Effect of Adding Different Concentrations of Cows’ Milk on the Antioxidant Properties of Coffee. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=22139 |
Introduction
Oxygen is an essential element for the survival of aerobic organisms. In many chemical and biochemical reactions, oxygen-derived free radicals are produced from oxidation-reduction processes.1 These free radicals are responsible for oxidative stress that leads to variety of diseases and disorders such as cancer,2 cardiovascular diseases,3 neural disorders,4 and Alzheimer’s disease.5 They are produced under normal physiological conditions and are generated by exogenous chemicals6. Reactive oxygen species (ROS) can present in different forms of activated oxygen such as hydrogen peroxide (H2O2) or as singlet oxygen.7 The redox homeostasis of cell is maintained by the antioxidant protection mechanism of the human body. If this mechanism has been unbalanced, the reducing of oxidative damage may be occurred by using antioxidant supplement.6
Coffee is an exogenous antioxidant that is widely consumed due to its desirable sensory properties.8 The proper consumption of coffee is important to health as many negative and positive effects can occur according to the dose. Negative effects of coffee consumption include hypertension, heart diseases, psychiatric disorders and bone diseases. These side effects are mainly attributed to increased caffeine consumption of coffee.9,10 On the other hand, a regular intake of coffee has shown many positive effects on metabolic, psychoactive and neurological diseases.11 The positive effects depend on the phenolic and flavonoid compounds such as chlorogenic acids (quinyl esters of hydroxycinnamic acid),12 caffeic, ferulic, p-coumaric acids13 and proanthocyanidins.13,14 These biological compounds are secondary metabolites from plants6 and have potent strong antioxidant and radical scavenging activities,15,16 which help the body to reduce the oxidative stress17
Most of people prefer to drink coffee with additives. Milk is one of the preferable choices of additives because it contains various compounds such as water, fats, sugars, minerals, vitamins, and proteins.18 When milk is added to coffee, the milk proteins (caseins and whey proteins) can interact with the coffee polyphenols and form a complex, which might affect many properties of coffee particularly the structural, functional, and nutritional ones.19-21 Many studies have investigated the antioxidant capacity of tea after adding different types of milk to it. However, the obtained results are contradictory. Some of them have reported an inhibitory effect, while others have shown no significant effect of adding milk.22-24 Milk fat exists in emulsified form coated by thin layer known as a globule membrane25 and its effect on antioxidant activity and bioavailability of coffee polyphenols is unclear until now. Therefore, the objective of this study was to assess the in vitro effect of adding different concentrations of whole, semi-skimmed, and skimmed cow’s milk on the antioxidant activity of coffee.
Materials and methods
Preparation of samples
Red mug coffee (Nescafé) and different types of long life (Almarai) cow’s milk (whole, semi-skimmed, and skimmed), the most popular consumed milk brand according to the survey, were purchased from a local supermarket. The types of milk and the percentages used were selected according to a survey performed on 900 participants in Jeddah, Western region of Kingdom of Saudi Arabia.
Coffee brews have been prepared as people in the survey commonly drink it. Each sample was prepared by adding 2g of instant coffee to 200 ml boiling water and then either 20 ml or 40 ml of different types of cow milk was added to have a final concentration of 10% or 20%, respectively.
Determination of total phenolic and flavonoid contents
In a clean test tube, 0.5ml of each coffee sample (with or without milk) was incubated at room temperature for 5 minutes with 5ml deionized water and 5ml Folin-Ciocalteu’s reagent (BDH, Poole, England). Then, in a dark place, all tubes were incubated with 1ml of anhydrous sodium carbonate (CDH, New Delhi, India) for 1 hour before the absorbance was measured at 750 nm. A standard curve was prepared from a serial concentration (0 to 1000µg/ml) of Gallic acid (Sigma-Aldrich, Poole, UK) which was prepared in methanol: water (50: 50 v/v).26,27
On the other hand, the total flavonoid content was determined by incubating 250μl of each coffee sample (with or without milk) with 1.25 ml deionized water and 75μl sodium nitrite (Sigma-Aldrich, Poole, UK) for 5 minutes. Then, 150μl of (10%) aluminum chloride (loba chemie PVT.LTD., Mumbai, India), 0.5 ml of (1M) sodium hydroxide (AppliChem Panreac, Missouri, USA) and 275μl of deionized water were added to the previous mixture. After that, the absorbance was read at 510 nm and a standard curve was prepared from a serial dilution (0 to 500µg/ml) of β-Catechin (Sigma-Aldrich, Poole, UK).28
Determination of radical scavenging activity with H2O2 and DPPH assays
In test tubes, 1 ml of sample was incubated for 10 minutes at room temperature with 0.6 ml of (40 mM) hydrogen peroxide (Sigma-Aldrich, Poole, UK). Then, the absorbance was recorded at 230 nm and the radical scavenging activity for H2O2 was calculated by the following equation: inhibition % = [(Absorbance of blank – Absorbance of sample) / Absorbance of blank] × 100.29,30
In the DPPH-radical scavenging assay, ethanol (Sigma-Aldrich, Poole, UK) and DPPH reagent (Sigma-Aldrich, Poole, UK) were added to water extract solutions of samples and were incubated in dark place for 1 hour at room temperature. The absorbance was measured at 517 nm and the DPPH radical scavenging activity was calculated using the same equation used to calculate the inhibition percentage of H2O2.31
Determination of metal chelating and reducing power activity
To estimate the ferrous ion chelating activity of samples,32 5µl of each sample (with or without milk) was incubated for 30 seconds with 50µl of (2mM) ferrous chloride (Sigma-Aldrich, Poole, UK) and 1.5ml distilled water. Then, 100µl of (5 mM) ferrozine (Sigma-Aldrich, Poole, UK) was added to samples and was incubated at room temperature for 10 minutes. The absorbance was measured at 562 nm and the following equation (Chelating Activity % = (Absorbance of control –Absorbance of sample)/Absorbance of control) ×100) was used to estimate the percentage of ferrozine-Fe2+ complex.
Regarding the reducing power assay,33 1ml of sample was mixed with phosphate buffer (0.2M, pH 6.6) (Oxoid, Hampshire, England) and potassium ferricyanide (1%) (Koch-light, Colnbrook Bucks, UK) before they were incubated in 50°C for 30 minutes. Then, trichloroacetic acid (TCA) (10%) (Sigma-Aldrich, Poole, UK) was added to stop reaction and then tubes were centrifuged at 6000 rpm for 10 minutes. The supernatant was mixed with equal amount of distilled water and ferric chloride (1%) (BDH, Poole, England) and the absorbance was immediately measured at 700 nm.
Statistical analysis
GraphPad Prism 7 software was used to analyze the results. Three independent experiments were performed for each assay and the means were assessed using one-way ANOVA test with Bonferroni’s test correction. The data were expressed as mean ± SD and results were considered statistically significant when P<0.05.
Results
Estimation of total phenolic and flavonoid contents
In this study, a survey was performed on 900 participants that live in Jeddah, Kingdom of Saudi Arabia to assess the most consumed coffee type, the average daily consumption of coffee-cups, and the type of additives used commonly when drink coffee. Results from survey revealed that (7%) of the participants prefer to add milk (with either 10% as minimum concentration or 20% as maximum concentration) to coffee. Therefore, this study was interested in studying the effect of adding different milk types with different concentrations on the phenolic compounds bioavailability and the antioxidant activities of coffee. To test all these objectives, many samples were prepared and used in this study [Nescafé red mug coffee, Nescafé red mug coffee with whole milk (10% and 20%), Nescafé red mug coffee with semi-skimmed milk (10% and 20%), and Nescafé red mug coffee with skimmed milk (10% and 20%)].
The total phenolic content was determined with Folin-Ciocalteu assay and was expressed as Gallic acid equivalent whereas; the total flavonoid content was determined with aluminum chloride assay and was expressed as Catechin equivalent. Table (1) represents the total phenolic and flavonoid contents in the samples. Generally, results showed that adding milk to coffee improves the contents of total phenolic and flavonoid compounds especially with the whole milk, either at 10% or 20% concentration.
Table 1: Total phenolic and flavonoid contents
| Samples | 10% Milk | 20% Milk | ||
| Total phenolic content (µg Gallic acid/2g coffee)* | Total flavonoid content (µg of Chatecin/2g coffee)* | Total phenolic content (µg of Gallic acid/2g coffee)* | Total flavonoid content (µg of Chatecin/2g coffee)* | |
| Coffee (no milk added) | 695 ± 2.5 | 506 ± 1.5 | 647 ± 3.4 | 506 ± 4.2 |
| Coffee with whole milk | 1181 ± 3.0 | 977 ± 6.0 | 1678 ± 7.0 | 1353 ± 5.0 |
| Coffee with semi-skimmed milk | 836 ± 5.0 | 822 ± 7.0 | 1061 ± 3.0 | 907 ± 2.5 |
| Coffee with skimmed milk | 732 ± 7.0 | 694 ± 5.0 | 897 ± 6.0 | 639 ± 5.0 |
*Data were represented as Mean±SD of three independent experiments.
Estimation of radical scavenging activity
To determine the radical scavenging activity of coffee samples, two assays were performed. The first assay, H2O2 radical scavenging assay, measured the ability of samples to scavenge hydroxyl radical at 320 nm. Hydrogen peroxide is one of the most reactive oxygen species (ROS) that can cross cell membranes rapidly.34 Once it is inside the cell, H2O2 can form hydroxyl radical through reaction with (Fe2+) and (Cu2+).35 Polyphenols of coffee can scavenge free radical by donating electron as illustrated in the equation:
2 H2O2→ 2 H2O + O2
In the second assay (DPPH radical scavenging assay), the scavenging reaction between (DPPH) and an antioxidant (H-A) can be expressed by the equation:
(DPPH) + (H-A) → DPPH-H + (A)
(Purple) (Yellow)
Antioxidants in coffee can react with DPPH and reduce it to the DPPH-H form (which can be determined by a reduction in the absorbance).36 Table 2 represents the results from H2O2 and DPPH radical scavenging assays. The results in Fig 1 showed that adding any type of milk (whole, semi-skimmed, and skimmed) at any concentration, 10% or 20%, had a paradoxical effect on the radical scavenging activity. For H2O2 scavenging activity, adding any type of milk at low concentration (10%) did not show any significant effect on the scavenging activity, whereas; at high concentration (20%), the activity of coffee to scavenge H2O2 reduced significantly when milk is added. In contrast, the addition of milk improves significantly the activity of coffee to scavenge any radicals (as shown by DPPH assay) at both concentration of milk (10% or 20%).
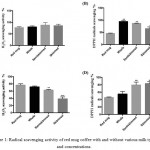 |
Figure 1: Radical scavenging activity of red mug coffee with and without various milk types and concentrations.
|
Three different types of milk were tested (whole, semi-skimmed, and skimmed) at two concentrations (10% and 20%). A & B) represents the H2O2 and DPPH radical scavenging activity of samples at 10% milk concentration, whereas; C & D) represents the H2O2 and DPPH radical scavenging activity of samples at 20% milk concentration. Results revealed that the addition of milk at any concentration or type decreases the H2O2 radical scavenging activity of the coffee, whereas; increases the DPPH radical scavenging activity. The values expressed as mean±SD (n=3). Comparisons of means were made using one-way ANOVA followed by a correction with Bonferroni’s test (*P<0.05,** P≤0.01, and *** P≤0.001).
Table 2: Estimation of the radical scavenging activity
| Samples | 10% Milk | |||
| H2O2 radical scavenging activity | DPPH radical scavenging activity | |||
| Mean ± SD | P-value | Mean ± SD | P-value | |
| Coffee (no milk added) | 77% ± 3.82 | REFERENCE | 45% ± 1.54 | REFERENCE |
| Coffee with whole milk | 80% ± 4.96 | P>0.05 (NS) | 94% ± 3.0 | ***P≤0.001 |
| Coffee with semi-skimmed milk | 88% ± 5.0 | P>0.05 (NS) | 84% ± 1.0 | **P≤0.01 |
| Coffee with skimmed milk | 85% ± 6.5 | P>0.05 (NS) | 65% ± 7.0 | *P≤0.05 |
| 20% Milk | ||||
| Samples | H2O2 radical scavenging activity | DPPH radical scavenging activity | ||
| Mean ± SD | P-value | Mean ± SD | P-value | |
| Coffee (no milk added) | 76% ± 3.0 | REFERENCE | 45% ± 1.5 | REFERENCE |
| Coffee with whole milk | 72% ± 2.0 | P>0.05 (NS) | 55% ± 7.0 | P>0.05 (NS) |
| Coffee with semi-skimmed milk | 62% ± 1.6 | **P≤0.01 | 80% ± 7.0 | **P≤0.01 |
| Coffee with skimmed milk | 38% ± 5.0 | ***P≤0.001 | 83% ± 5.0 | **P≤0.01 |
-All data were represented as Mean ±SD calculated by one-way ANOVA test followed by Bonferroni’s test correction.
-P-values were calculated by a comparison made versus the (coffee only) value, which was referring to it as reference.
NS: Not Significant
Estimation of metal chelating and reducing power activity
Two assays were performed to estimate the ability of antioxidants, present in the samples, to act as metal chelating agents and as reducing agents. In ferrous chelating activity assay, the dark color which is formed by the interaction of ferrozine with Fe2+ is changed into a lighter color in the presence of a chelating agent in the sample.37 On the other hand, ferric reducing power assay used the Fe3+ reduction as an indicator of electron-donating activity which is an important mechanism of any phenolic antioxidant.38
Table 3 represents the results from metal chelating and reducing power assays. Interestingly, the results in Fig 2 showed that adding any type of milk (whole, semi-skimmed, and skimmed) at any concentration, either 10% or 20%, reduces significantly the metal chelating and the metal reducing power activities of the coffee compared to the coffee alone (with no milk added).
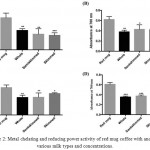 |
Figure 2: Metal chelating and reducing power activity of red mug coffee with and without various milk types and concentrations.
|
Three different types of milk were tested (whole, semi-skimmed, and skimmed) at two concentrations (10% and 20%). A & B) represents the metal chelating and reducing power activity of samples at 10% milk concentration, whereas; C & D) represents the metal chelating and reducing power activity of samples at 20% milk concentration. Results revealed that the addition of milk at any concentration or type decreases the metal chelating and reducing power activity of the coffee. The values expressed as mean±SD (n=3). Comparisons of means were made using one-way ANOVA followed by a correction with Bonferroni’s test (*P<0.05,** P ≤0.01, and *** P ≤0.001).
Table 3: Estimation of metal chelation and reducing power activity
| Samples | 10% Milk | |||
| Ferrous chelating activity | Ferric reducing power activity | |||
| Mean ± SD | p-value | Mean ± SD | P-value | |
| Coffee (no milk added) | 37% ± 5.50 | REFERENCE | 0.62 ± 0.05 | REFERENCE |
| Coffee with whole milk | 23% ± 1.89 | **P≤0.01 | 0.38 ± 0.03 | **P≤0.01 |
| Coffee with semi-skimmed Milk | 18% ± 1.42 | **P≤0.01 | 0.43 ± 0.09 | *P<0.05 |
| Coffee with skimmed milk | 17% ± 2.84 | ***P≤0.001 | 0.42 ± 0.06 | *P<0.05 |
| 20% Milk | ||||
| Samples | Ferrous chelating activity | Ferric reducing power activity | ||
| Mean ± SD | P-value | Mean ± SD | P-value | |
| Coffee (no milk added) | 38% ± 3.40 | REFERENCE | 0.61 ± 0.04 | REFERENCE |
| Coffee with whole milk | 22% ± 2.80 | **P≤0.01 | 0.35 ± 0.02 | ***P≤0.001 |
| Coffee with semi-skimmed milk | 23% ± 5.40 | **P≤0.01 | 0.37 ± 0.02 | ***P≤0.001 |
| Coffee with skimmed milk | 27% ± 0.86 | *P<0.05 | 0.33 ± 0.04 | ***P≤0.001 |
-All data were represented as Mean ±SD calculated by one-way ANOVA test followed by Bonferroni’s test correction.
-P-values were calculated by a comparison made versus the (coffee only) value, which was referring to it as reference.
Discussion
Coffee is one of the most popular beverages in the world. Many of the studies indicated that adding some additives to coffee can alter the bioactive compounds present in it, and therefore, may affect the biological activity of coffee as an important antioxidant. In many cases, coffee is consumed with milk. Milk protein may form interaction with coffee polyphenols, therefore, the present study aimed to investigate the total phenol and flavonoid contents and the antioxidant activity of bioactive components when different types of milk (whole, semi-skimmed, and skimmed) at both concentration (10% and 20%) were added to coffee. Our results indicated that adding milk to coffee increased the total phenol and flavonoid contents. This might be explained by the presence of other compounds in milk that can act as an antioxidant such as lactoferrin, ascorbic acid, tocopherols and tocotrienols.39
Hydrophobic interaction and hydrogen bond are the major reactions that derive polyphenol-protein complex formation. Several factors can influence the extent of these binding forces such as structure of polyphenols and proteins as well as some physical conditions particularly (temperature, pH, and ionic strength).40 In our study, our results revealed that at 10% milk concentration, there was no significant difference in hydrogen peroxide scavenging activity between the coffee with and without milk. Interestingly, adding milk at higher concentration (20%) decreased the H2O2 scavenging activity for coffee especially with skimmed milk. Some flavonoids have a high affinity to bind to proteins.40 This affinity makes many phenol groups of flavonoids occupied and thus H2O2 scavenging do not occur properly. The formation of polyphenol-protein complex can result in protein unfolding. In the presence of lipids, which are classified as Amphiphilic molecules, hydrophobic lipid phase interacts with hydrophobic part of the protein, which has been exposed to the outer phase due to denaturation. This prevents the association between hydrophobic sites of polyphenols with the aromatic groups and aliphatic side chains of hydrophobic amino acids.41 However, those polyphenols when bind with the polar head groups of lipid can probably make phenol groups somehow in a position that allows phenols to scavenge H2O2, while polyphenol-protein complex without lipid presence require the involvement of many hydrogen and hydrophobic bonds in addition to the high affinity of flavonoids toward protein binding.40 These findings may explain why whole milk sample has H2O2 scavenging activity higher than semi-skimmed and skimmed milks.
On other hand, in the present study, results from DPPH radical scavenging activity experiments are in contrast with that of H2O2 for all tested samples. In addition to the probabilities that mentioned above about the difference of phenols, some amino acids with aromatic and bulky side groups are considered as effective radical scavengers because they can act as H-donors due to the presence of special groups in their side chains. For example, the phenolic group in tyrosine, indolic group in tryptophan, imidazole group in histidine, and sulfur hydrogen42 in methionine and cysteine. Moreover, several studies refer to the correlation between the high concentration of flavonoids and polyphenols with the high DPPH scavenging activity.43,44 In our study, the polyphenol and flavonoid contents were higher in all coffee with milk samples compared to the plain coffee sample.
In general, many studies, reported that the polyphenols interaction with proteins, not only affects the scavenging activity but also affects the chelating and metal reducing activity of coffee.18,45 The hydrophobic stacking of the aromatic groups of the protein and polyphenols, or the interaction of the –OH groups of the polyphenols with the protein chain can resulted in the formation of aggregates.40 Our results showed that the ferrous chelating activity increased when coffee is not mixed with milk. This might be due to the presence of free –OH groups of polyphenols or might be related to the binding of polyphenols to the milk caseins by covalent and non-covalent interactions either in a multi-site or in a multi-dentate interaction.24 Our results are in agreement with other similar studies22,46 that used FRAP assay to assess the ferrous chelating activity.
In conclusion, the results of the current study revealed that milk might either enhance the scavenging of DPPH or decrease the chelating and metal reducing activity of polyphenol. However, more investigations are required to evaluate the mechanisms by which fats in milk can alter the antioxidant properties of coffee.
Acknowledgements
This research was supported by the Science Research & Innovation Unit at the Faculty of Science, King Abdulaziz University in Jeddah, Saudi Arabia.
Footnotes
Authors’ contribution
Ulfat M. Omar developed the original idea and performed the statistical analysis. Ayat Badr Al-Ghafari and Huda A. Al Doghaither prepared the manuscript. Rahaf H. Alharbi, Manal M. Al-Jehani, and Shoroq A. Bujeir performed the experiments.
References
- Mohan S. C., Balamurugan V., Salini S. T., Rekha R. Metal ion chelating activity and hydrogen peroxide scavenging activity of medicinal plant Kalanchoe pinnata. Chem. Pharm. Res. 2012;4(1):197–202.
- Kinnula V. L., Crapo J. D. Superoxide dismutases in malignant cells and human tumors. Free Radic. Biol. Med. 2004;36(6):718–44.
CrossRef - Singh U., Jialal I. Oxidative stress and atherosclerosis. Pathophysiology. 2006;13(3):129–42.
CrossRef - Sas K., Robotka H., Toldi J., Vécsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. Neurol. Sci. 2007;257(1-2):221–39.
CrossRef - Smith M. A., Rottkamp C. A., Nunomura A., Raina A. K., Perry G. Oxidative stress in Alzheimer’s disease. Biophys. Acta.(BBA)- Molecular Basis of Disease. 2002;1502(1):139–44.
CrossRef - Chanda S., Dave R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties : An Afr. J. Microbiol. Res. 2009;3(13):981–96.
- Halliwell B. How to characterize an antioxidant: an update. Soc. Symp. 1995;61:73–101.
CrossRef - Camargo M. C., Toledo M. C., Farah H. G. Caffeine daily intake from dietary sources in Brazil. Food Addit. Contam. 1999;16(2):79–87.
CrossRef - Nawrot P., Jordan S., Eastwood J., Rotstein J., Hugenholtz A., Feeley M. Effects of caffeine on human health. Food Addit. Contam. 2003;20(1):1–30.
CrossRef - Smith P., Smith A., Miners J., Mcneil J., Proudfoot A. The safety aspects of dietary caffeine. Food Standards Australia New Zealand. 2000.
- Dórea J. G., da Costa T. H. M. Is coffee a functional food? J. Nutr. 2007;93(06):773–82.
CrossRef - Olthof M. R., Hollman P. C., Zock P. L., Katan M. B. Consumption of high doses of chlorogenic acid, present in coffee, or of black tea increases plasma total homocysteine concentrations in humans. J. Clin. Nutr. 2001;73(3):532–38.
- Richelle M., Tavazzi I., Offord E. Comparison of the antioxidant activity of commonly consumed polyphenolic beverages (coffee, cocoa, and tea) Prepared per Cup Serving. Agric. Food Chem. 2001;49(7):3438–42.
CrossRef - Arts I. C. W., van de Putte B., Hollman P. C. H. Catechin contents of foods commonly consumed in the Netherlands. 2. tea, wine, fruit juices, and chocolate milk. Agric. Food Chem. 2000;48(5):1752–57.
CrossRef - Cämmerer B., Kroh L.W. Antioxidant activity of coffee brews. Food Res. Technol. 2006;223(4):469–74.
CrossRef - Parras P., Martineztome M., Jimenez A., Murcia M. Antioxidant capacity of coffees of several origins brewed following three different procedures. Food Chem. 2007;102(3):582–92.
CrossRef - Serafini M., Testa M. F., Villano D., Pecorari M., van Wieren K., Azzini E., Brambilla A. Maiani G. Antioxidant activity of blueberry fruit is impaired by association with milk. Free Radic. Biol. Med. 2009;46(6):769–74.
CrossRef - Ozdal T., Capanoglu E., Altay F. A review on protein-phenolic interactions and associated changes. Food Research International. 2013;51(2):954–70.
CrossRef - Charlton A. J., Baxter N. J., Khan M. L., Moir A. J. G., Haslam E., Davies A. P., Williamson M. P. Polyphenolpeptide binding and precipitation. Agric. Food Chem. 2002;50(6):1593–1601.
CrossRef - Spencer C. M., Cai Y., Martin R., Gaffney S. H., Goulding P. N., Magnolato D., Lilley T. H., Haslam E. Polyphenol complexation—some thoughts and observations. Phytochemistry. 1988;27(8):2397–2409.
CrossRef - Yuksel Z., Avci E., Erdem Y. K. Characterization of binding interactions between green tea flavanoids and milk proteins. Food Chem. 2010;121(2):450–56.
CrossRef - Langley-Evans S. C. Antioxidant potential of green and black tea determined using the ferric reducing power (FRAP) assay. J. Food Sci. Nutr. 2000;51(3):181–88.
CrossRef - Reddy V. C., Vidya Sagar G. V., Sreeramulu D., Venu L., Raghunath M. Addition of milk does not alter the antioxidant activity of black tea. Nutr. Metab. 2005;49(3):189–195.
CrossRef - Sharma V., Kumar V. H., Rao L. M. J. Influence of milk and sugar on antioxidant potential of black tea. Food Res. Int. 2008;41(2):124–29.
CrossRef - Rashidinejad A., Birch E. J., Sun-Waterhouse D., Everett D.W. Addition of milk to tea infusions: Helpful or harmful?; evidence from In vitro and In vivo studies on antioxidant properties. Rev. Food Sci. Nutr. 2015. http://dx.doi.org/10.1080/10408398.2015.1099515.
CrossRef - Singleton V. L., Orthofer R., Lamuela-Raventós R. M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods. 1999;299:152–178.
CrossRef - Kähkőnen M. P., Hopia A. I., Vuorela H. J., Rauha J. P., Pihlaja K., Kujala T. S., Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. Agric. Food Chem. 1999;47(10):3954–62.
CrossRef - Ayuko T. A., Njau R. N., Cornelius W., Leah N., Ndiege I. O. In vitro antiplasmodial activity and toxicity assessment of plant extracts used in traditional malaria therapy in the Lake Victoria Region. Inst. Oswaldo Cruz. 2009;104(5): 689–94.
CrossRef - Ruch R. J., Cheng S., Klaunig J. E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10(6):1003–8.
CrossRef - Gűlçin I., Huyut Z., Elmastas M., Aboil-Enein H. Radical scavenging and antioxidant activity of tannic acid. J. Chem. 2010;3(1):43–53.
CrossRef - Bersuder P., Hole M., Smith G. Antioxidants from a heated histidine glucose model system I: Investigation of the antioxidant role of histidine and isolation of antioxidants by high performance liquid chromatography. Am. Oil Chemists. Soc. 1998;75:181–87.
CrossRef - Dinis T. C. P., Madeira V. M. C., Almeida L. M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Biochem. Biophys. 1994;315(1):161–69.
CrossRef - Al-Ghafari A. B., Shorbaji A. M., Baduwailan E. O., Basaar A. A., Al Doghaither H. A., Al, Al-marzouki H. F., Omar U. M. Phenolic contents and antioxidant activities of green tea with and without lemon. Sci. 2016;8:247–55.
- Keser S., Celik S., Turkoglu S., Turkoglu I. Hydrogen peroxide radical scavenging and total antioxidant activity of Hawthorn. Chem. J. 2012;2(1):9–12.
- Priyanka B., Anitha K., Shirisha K., Sk J., Dipankar B., Rajesh K. Evaluation of antioxidant activity of ethanolic root extract of Albizia. IRJPAS. 2013;3(2):93–101.
- Arulpriya P., Lalitha P. Hemalatha, S. In vitro antioxidant testing of the extracts of Samanea saman (Jacq.) Merr. Der Chemica Sinica. 2010;1(2):73–79.
- Geckil H., Ates B., Durmaz G., Erdogan S., Yilmaz I. Antioxidant, free radical scavenging and metal chelating characteristics of propolis. J. Biochem. Biotechnol. 2005;1(1):27–31.
CrossRef - Yildirim A., Mavi A., Bo B. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. Food Chem. 2001;49(8):4083–89.
CrossRef - Lindmark-Månsson H., Akesson B. Antioxidative factors in milk. J. Nutr. 2000;84:S103–S110.
CrossRef - Bandyopadhyay P., Ghosh A. K., Ghosh C. Recent developments on polyphenol–protein interactions: effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. 2012;3:592–605.
CrossRef - Dubeau S., Samson G., Tajmir-Riahi H. A. Dual effect of milk on the antioxidant capacity of green, Darjeeling, and English breakfast teas. Food Chem. 2010;122(3):539–45.
CrossRef - Ajibola C. F., Fashakin J. B., Fagbemi T. N., Aluko R. E. Effect of peptide size on antioxidant properties of African yam bean seed (Sphenostylis stenocarpa) protein hydrolysate fractions. J. Mol. Sci. 2011;12(10):6685–6702.
CrossRef - Aoshima H., Ayabe S. Prevention of the deterioration of polyphenol-rich beverages. Food Chem. 2007;100(1):350–55.
CrossRef - Ebrahimzadeh M. A., Nabavi S. M., Nabavi S. F., Bahramian F., Bekhradnia A. R. Antioxidant and free radical scavenging activity. J. Pharm. Sci. 2010;23(1):29–34.
- Jakobek L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015;175:556–67.
CrossRef - Niseteo T., Komes D., Belščak-Cvitanović A., Horžić D., Budeč M. Bioactive composition and antioxidant potential of different commonly consumed coffee brews affected by their preparation technique and milk addition. Food Chem. 2012;134(4):1870–77.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





