Manuscript accepted on : 21 February 2017
Published online on: --
Plagiarism Check: Yes
Shetta, N. D.1, 2
1Department of Forestry and Wood Technology, Faculty of Agriculture, Alexandria University, Alexandria, Egypt.
2Plant Production Dept., Food and Agricultural Sciences College, King Saud University, Riyadh, Saudi Arabia.
Corresponding Author E-mail: n.shetta@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2440
ABSTRACT: Begin with the influence of different mineral fertilizers (P:K 1:1 and NPK 1:1:1) on nodulation and growth of two introduced Acacia species grown under nursery was investigated. Two Acacia Acacia ampliceps and A. salicina were treated with combinations of N, P, and K fertilizers and two inoculation (seed inoculation and soil inoculation). Nodulation status (nodule number and nodules dry weight) in roots and plant growth parameters (seedling length, collar diameter, and shoot and root dry weight) were recorded 120 days after inoculation. Soil inoculation with Rhizobium in combination with mineral fertilizer) significantly affected growth and nodulation compared with soil inoculations alone. Seeds inoculated with PK+ Rhizobium performed better than other treated seeds and resulted in seedlings with the most growth and root nodules. The Rhizobium strain LLR14 was highly effective in both Acacia seedlings. Acacia ampliceps was more responsive than A. salicina to Rhizobium inoculation in combination with mineral fertilizer treatments. The use of fertilizers and inoculation in the nursery may facilitate the production of superior seedlings with high growth potential, reducing the time needed to achieve canopy closure. A. ampliceps and A. salicina seedlings inoculated with Rhizobium and treated with P or K fertilizer may facilitate afforestation and reforestation programs, particularly in the arid regions.
KEYWORDS: Introduced Acacia species; Inorganic fertilizer; Inoculation methods; Nursery conditions
Download this article as:| Copy the following to cite this article: Shetta, N. D.Influence of Minerals Fertilizer Addition and Inoculation Methods on Nodulation and Growth of Two Introduced Acacia species. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Shetta, N. D. Influence of Minerals Fertilizer Addition and Inoculation Methods on Nodulation and Growth of Two Introduced Acacia species Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=21886 |
Introduction
Chemical fertilizers have been used for plant cultivation because of their high solubility and positive impacts on crop yield. Inorganic fertilizers may influence plant adaptation and their ability to dominate and flourish in legume production.1,2 Begin with ” Mineral” fertilizers are also an important factor in modern agriculture; approximately 60% of humanity currently owes its nutritional survival to nitrogen (N) fertilizers. Growing concern about the environmental consequences of mineral N use emphasize the need to develop new production technologies that are sustainable for economically and ecologically.3
While application of N fertilizers to seedlings can increase N2 fixation through a starter effect, such a method is costly; with high ATP needs, N2 fixation generally has a high requirement for the mineral phosphorus (P) and P adjustments on low-P soil increases biological N fixation rates.4,5 Conversely, N2-fixing tree species may compete with non-fixing species for P in afforestation, ultimately reducing soil P availability 6 and necessitating requiring additional P adjustments. Although the addition of P to P-poor soils usually lead to increased N2 fixation, this has sometimes also encouraged tree growth; in dryland West Africa, for example, as little as 30 kg P ha-1 enhanced the growth of woody legumes.4
Acacia ampliceps (Maslin.) and Acacia salicina (Lindley) belong to the legume family. They are thornless species native to Australia that were introduced to Saudi Arabia in the 1980s. Both species grow as fast-growing dense shrubs or small trees (5–20 m tall) with a spreading crown. They are able to grow in the arid and semi-arid regions of Saudi Arabia They are are considered to be one of the most drought- and salt-tolerant species that are able to grow on a wide range of soils. Furthermore, these Acacia species are highly adapted to low rainfall and high temperature conditions, allowing them to grow in degraded soils with poor fertility levels 7. These two Acacia species are found on sand plains, flood plains, and along drainage lines and are important sources of fodder, fuel, and timber.8,9.10 They can form a symbiotic association with rhizobia that encourages the formation of nodules where atmospheric N is fixed.11 These two species were selected for their prominence in the afforestation and reforestation of the central region of Saudi Arabia.
The importance of woody-legume–Rhizobium symbioses, which are able to fix considerable quantities of N2 and serve as a cheap fertilizer replacement, has been frequently reported for land reclamation and land improvement, particularly in arid regions with high salinity, low fertility, and drought periods. The majority of leguminous plant form symbiotic relationships with members of genera that belong to the class Alphaproteobacteria (Allorhizobium, Azorhizobium, Blastobacter, Bradyrhizobium, Devosia, Ensifer, Mesorhizobium, Methylobacterium, Rhizobium, and Sinorhizobium). Some legumes, such as those belonging to the Mimosa genus, are nodulated mostly by members of the class Betaproteobacteria in the genera Burkholderia and Cupriavidus. Recently, the endemic Mexican mimosas were shown to be nodulated predominantly by Alphaproteobacteria from the genera Rhizobium and Ensifer.12,13 Work on Rhizobium-legume symbiosis has been conducted over many years; several studies examining rhizobia isolated from tree legumes have indicated that there is considerable phenotypic and genotypic diversity among strains, although less attention has been given to interaction symbionts of introduced Acacia species.14 Acacia species can assist understory plants by N fixation.15,16 The growth of understory plants will develop the plant community by modifying physical and chemical properties of the soil beneath the canopy.17
Inoculation of legume seeds is an efficient way to introduce effective Rhizobium to the soil and to the rhizosphere of legumes.18 The efficacy of inoculation varies depending the number of viable Rhizobium available to infect the legume roots. Previous studies in nursery conditions have indicated that it was possible to improve the growth of Acacia species by inoculating them with effective micro symbionts.19 Inoculation does result in higher numbers of viable rhizobia on seeds and there is substantial demand for commercially inoculated legume seeds. There are a number of different methods that can be used depending on the seed size and availability of equipment. Sarr et al.20 demonstrated that the improvement in growth of Acacia species was more marked if the inoculation of trees was carried out using dissolved alginate beads containing a mixture of selected rhizobial strains; there is, however, no information regarding such growth responses in natural environments. Many factors in the field are related to the success of the rhizobia inoculation, including drought, soil fertility, the genetic source of the host plant, and the ability of rhizobia present in the soil to infect the host plant in the presence of host-compatible native rhizobial strains.21 Native strains of rhizobia can negatively affect inoculation and the majority of isolates forming nodules on field-grown plants are usually relatively poor N fixers.22 The competitiveness of a rhizobial strain contained in an inoculum in terms of host-plant nodulation must be assessed before unsterilized soil containing a large population of native rhizobia compatible with the target host plant is inoculated. Under greenhouse conditions, Lesueur and Diouf 23 showed significant growth response differences between two variants of Calliandra calothyrsus inoculated with a rhizobial strain. Likewise, Sarr et al.20 demonstrated a strong interaction effect on tree growth between A. senegal and A. nilotica with a mixture of rhizobial strains.
The objective of the current study was to establish guidelines to examine and optimize root nodule formation in two introduced Acacia trees by investigating the inoculation methods of Rhizobium in conjunction with subsequent mineral fertilizer treatments, ensuring high growth rates in individual trees under Riyadh region conditions.
Materials and Methods
Experimental procedures
This study was carried out at the nursery of the Range and Forestry Applied Research Unit at the Experimental Station of the Food and Agricultural College at Dirab during the two growth seasons of 2015 and 2016. Seeds of A. ampliceps (Maslin.) and A. salicina (Lindley) used in this study were obtained from the Range and Forestry Applied Research Unit, and Dirab Valley, South of Riyadh City. The characteristics of the soil used in the experiments are shown in Table(1).
Table 1: Physical and chemical characteristics of the soil used in the study
| Particle size distribution (%) | Soil texture | pH | EC†
(ds m-1) |
Soluble cations
(mg L-1) |
Soluble anions
(mg L-1) |
Available nutrients
(mg kg-1) |
Organic Mater
OM % |
||||||
| sand | silt | clay | Na+ | K+ | Ca2+ | SO4-2 | CL– | N | P | 1.0 | |||
| 78.7 | 14.0 | 7.3 | Sandy loam | 8.65 | 1.45 | 1.04 | 0.15 | 6.10 | 6.0 | 2.2 | 13.0 | 0.15 | |
†EC: electrical conductivity
Mineral fertilizers and inoculation Methods
Three common mineral fertilizers, ammonium nitrate (33% N) as a source of N, 0.3 g/plant; superphosphate (15% P2O5) as a source of P, 0.33 g/plant; and potassium sulfate (50% K2O) as a source of K, 0.1 g/plant ,in addition to two different mix fertilizer treatments; PK 1:1 and NPK 1:1:1. were used in the study. For each fertilizer treatment, the dose was divided into two parts, the first dose administered in June and the second in July in each growing season. Two inoculation methods were used to infect the Acacia seedlings; a; seed inoculation by adding a 250 ml of bacteria mixture with sugar solution, and activated charcoal, conducted during germination in March, b; soil inoculation, where the soil of each plant was injected with 15 ml of rhizobium after seed germination
Rhizobium Strain used
Seedlings of A. ampliceps (Maslin.) and A. salicina (Lindley) were inoculated with strain Rhizobium strain (LLR14) isolated from the roots of Leucaena leucocephala trees grown in forestry nurseries at the Experimental Station of the Food and Agricultural College at Dirab. Bacteria were isolated from surface-sterilized nodules using the standard procedure of Vincent24 and cultured in yeast extract–mannitol agar (YMA) medium. All the isolates were subcultured and then incubated on YMA medium at a 30° angle and kept at 4°C; isolates were stored long-term in 20% glycerol at −80°C. The strain used in the study (Table 2) was a fast-growing type belonging to the Sinorhizobium fredii strain NGR 234 (data not published).
Table 2: Characterization of nodule-forming isolates from Leucaena leucocephala
| Host plant | Strain | Source of Strain | Growth period (hours) | Generation time (hours) | Rhizobium | Identity | |
| Query (bp) | Locus | ||||||
| Leucaena leucocephala | LLR14 | Wadi Dirab | 3–5 | 2.15 | Sinorhizobium fredii | 899 | CP001389 |
Seedling production and infection assay
Seeds of A. ampliceps and A. salicina were soaked in hot water (100°C) for 15 min and cool water (20°C) for 24 h, to prevent the inhibitory effect of seed coats on germination. Seeds of A. ampliceps and A. salicina then sown in sterilized medium (autoclaved at 121°C for 1 h) containing a mixture of sand and vermiculite (2:1 v/v) in the greenhouse, maintained at day/night temperatures of approximately 25°C/17°C. After germination, the seedlings were transplanted into pots (15 cm diameter) containing 2 kg of mixed sterilized sand and vermiculite. Pots were organized in a split-split plot in a randomized complete block design in a greenhouse . The Acacia seedlings were inoculated with 15 ml of mature Rhizobium isolates (LLR14; approximately 1010 bacterial cells ml/plant) grown in yeast extract mannitol broth culture incubated at 28°C with shaking (200 rpm) for 5 days. Two methods of infection were used to inoculate the seedlings, seed inoculation and soil inoculation.
The seedlings were placed in a greenhouse between March 2015 and the first week of May 2016. The daily maximum temperature ranged from 25 to 30°C and the minimum temperature from 19 to 20°C. At the treatments used in the study included; a) control treatment; b) inoculation with Rhizobium only using seed inoculation and/or soil inoculation; c) inoculation with Rhizobium using seed inoculation and/or soil inoculation with treatment with PK 1:1 fertilizer; and d) inoculation with Rhizobium using seed inoculation and/or soil inoculation with treatment with NPK 1:1:1 fertilizer. Seedlings were maintained in the greenhouse during the experiment and were watered every other day with tap water. Seedlings were harvested 120 days after planting, and the following measurements were recorded: seedling height and diameter shoot and root dry matter per seedling, number of nodules per seedling, and nodule dry weight per seedling. Seedlings were dried at 70°C and the macro and micronutrients were measured. Total N content was determined using the Kjeldahl method. K+ was determined using flame photometry (Corning 400, Sherwood Scientific Ltd, Cambridge, UK), and P was measured using colorimetric determination.
The Experimental Design
The split-split plot system in a randomized complete block design was used in this experimental according to Steel and Torrie 25; the main plot was the tree species, the subplot was fertilizer treatments, and the sub-subplot was Rhizobium isolates. Three replicates were used for each treatment. Statistical analysis was done using ANOVA, F-tests, and Least significant differences available within the SAS software package program26 (version 9.13 2008). The combined analysis was used for the two growing seasons.
Results and Discussion
Growth Parameters
The average seedlings height of A. ampliceps was taller than A. salicina ( Table 3). ampliceps seedlings were taller on average than A. salicina seedlings (Table 3). The most effective treatment was PK fertilizer in combination with average 19..83 cm followed by NPK with Rhizobium whereas the control resulted in shorter seedlings with an average height of 6.64 cm (Table 3). On the other hand, no significant differences (p <0.05) in growth height were found between the two inoculation methods (Table 4).
Table 3: Mean growth parameter values of tree species treated with fertilizers and Rhizobium over two growing seasons
| Tree species | Treatments | Growth parameters | Nodulation | ||||
| Height (cm) | Diameter (cm) | Shoot dry weight (g) | Root dry weight (g) | Number of nodules | Nodules dry weight (g) | ||
| Acacia ampliceps | Control | 6.64b | 0.08b | 3.30d | 1.29d | 0.0d | 0.0b |
| Rhizobium | 9.15b | 0. 08b | 12.54c | 10.45c | 37.2b | 0.3a | |
| PK+ Rhizobium | 19.83a | 0.17a | 35.49b | 17.17a | 46.9a | 0.4a | |
| NPK+ Rhizobium | 17.37a | 0.12b | 43.10a | 12.88b | 10.3c | 0.1a | |
| Mean | 13.45 | 0.11 | 23.61 | 10.45 | 23.61 | 0.2 | |
| Acacia salicina | Control | 5.86b | 0.04b | 2.21b | 0.8c | 0.0d | 0.0b |
| Rhizobium | 6.17b | 0.06a | 3.79b | 1.95b | 22.1b | 0.12a | |
| PK+ Rhizobium | 13.92a | 0.09a | 21.82a | 14.11a | 31.4a | 0.25a | |
| NPK+ Rhizobium | 12.43a | 0.10a | 21.60a | 13.23a | 8.6c | 0.06a | |
| Mean | 9.74 | 0.72 | 12.36 | 7.51 | 15.25 | 0.11 | |
The analysis of variance of the stem diameter showed highly significant differences (p <0.05) between tree species and fertilizer treatment while no significant differences were found between inoculation methods. The average stem diameter indicated that A. ampliceps seedlings had smaller stem diameters than A. salicina seedlings. The fertilizer treatments showed that PK with Rhizobium resulted in the widest stem diameter followed by NPK with Rhizobium (Table 3). The stem diameter results a indicated that the Pk and NPK treatments had different effects on the stem diameter; PK treatment with Rhizobium resulted in a larger average of stem diameter in A. ampliceps, while NPK treatment
resulted in a larger stem diameter in A. salicina in both growing seasons. The shoot and root dry weight varied between the two Acacia species whereas the inoculation method did not significantly affect the measured growth parameters.
For the shoot and root dry weight, highly significant differences (p <0.05) were found between tree species, fertilizer treatments, and their interactions over both growing seasons. A. ampliceps seedlings had higher shoot and root dry weights with either PK or NPK fertilizer in combination with Rhizobium than A. salicina (Table 3). The shoot and root dry weight varied between the two inoculations methods in the Acacia species. Soil inoculation resulted in higher shoot dry weight in A. ampliceps, whereas shoot dry weight of A. salicina was higher with the seed inoculation method. Conversely, the root dry weight was higher with seed inoculation in A. ampliceps and with soil inoculation in A. salicina (Table 4).
Table 4: Mean growth parameter values of tree species inoculated with Rhizobium using two different methods in combination with the fertilizer treatments across the two growing seasons
| Tree species | Inoculation method | Growth parameters | Nodulation | ||||
| Height (cm) | Diameter (cm) | Shoot dry weight (g) | Root dry weight (g) | Number of nodules | Nodules dry weight (g) | ||
| Acacia ampliceps | Seed inoculation | 7.95a | 0.08a | 12.79a | 8.0a | 18.58a | 0.21a |
| Soil inoculation | 7.33a | 0.08a | 13.98a | 6.79a | 6.75b | 0.06b | |
| Mean | 7.64 | 0.08 | 13.39 | 7.40 | 12.67 | 0.14 | |
| Acacia salicina | Seed inoculation | 9.55a | 0.05a | 9.40a | 4.95a | 15.0a | 0.05a |
| Soil inoculation | 8.16a | 0.06a | 8.0a | 5.04a | 8.1b | 0.05a | |
| Mean | 8.86 | 0.06 | 8.7 | 5.0 | 11.6 | 0.05 | |
The results from the two seasons indicated that A. ampliceps was more responsive to fertilizer treatment and Rhizobium inoculation method than A. salicina. Treatment with PK fertilizer and Rhizobium was the most effective compared with used in this study. Therefore, the findings of this study
are consistent with the results obtained by Ahmadi and Chaichi,27 Bekere and Hailemariam28 and Huda et al. 5, who showed that, the inoculation in combination with chemical fertilizer application resulted in greater concentrations of the nutrients and increased plant growth. The application of fertilizer significantly affected every measured parameter; fertilized seedlings were significantly taller.29,30 Inoculation with Rhizobium in addition to the application of fertilizer further improved the measured growth parameters. This result was consistent with the results obtained by Shetta,31 who found that the native Rhizobium increased the height growth of A. karroo, but contradicted with the results obtained by Sanchez et al.,32 who indicated that native Rhizobium populations were not effective enough to obtain a significant increase in shoot height and nodulation. On Contrary Ahmad et al.,33 found that seed inoculation with Rhizobium significantly increased plant height in many legumes.
The fertilizer rate significantly affected root diameters of Acacia koa.29 Moreover, the stem diameter increased following inoculation with Rhizobium strains using the soil inoculation method compared to the seed inoculation method. The inoculation efficiency varied according to several factors that affected the number of viable rhizobia available for root legume infection.18 In this study, inoculation with Rhizobium was found to increase the root diameter. This was consistent with the findings of Deaker et al.18 and Molla et al.,34 who indicated that the root growth and nodulation were significantly increased, but contradicted with the results obtained by Philpotts35. The seed inoculated plants exhibited significantly greater root and shoot mass as compared with the control plants. Seed inoculation has, however, been shown to result in decreasing bacterial rates.36,33 The enhancement of growth parameters observed following inoculation. in this study the results were consistent with the results obtained by Shaheen and Rahmatullah,37 Bekere and Hailemarion28,and Ali et al. 38 who showed that seed and soil inoculations combined with fertilizer treatments increased roots and shoots dry matter of legumes.
The Nodulation of Acacia Species
The results obtained in the present study indicated that both the Acacia species were able to form nodules with Rhizobium strains under all fertilizer treatments except the control treatment. The nodule number and nodule dry weight analyses revealed that highly significant differences (p <0.05) were found between tree species, fertilizer treatments, inoculation methods and their interactions. A. ampliceps was more responsive to fertilizer treatments and Rhizobium inoculation than A. salicina. Furthermore, the PK fertilizer treatment in conjunction with Rhizobium inoculation resulted in a higher number of nodules when compared with other fertilizer treatments in both Acacia species (Table 3). The effect of inoculation method on nodule number indicated that the seed inoculation method was more effective than the soil inoculation method in both A. ampliceps and A. salicina over the two growing seasons (Table 4).
Consistently, the nodule dry weight of A. ampliceps was higher than A. salicina in both growing seasons. Similarly, the PK fertilizer treatment in conjunction with Rhizobium inoculation resulted in higher nodule dry weight compared with NPK in both Acacia species in both Acacia species (Table 3). The inoculation method showed that seed inoculation was more effective in terms of increasing the nodule dry weight than the soil inoculation inoculation method in A. ampliceps; in A. salicina no differences were observed between the two inoculation methods (Table 4).
The seed inoculation method had a greater effect on root nodules and the dry weight of nodules than soil inoculation. The results obtained from our results are consistent with the results of previous findings of Huda et al.5 and Bekere and Hailemariam28 who indicated that fertilizer with P and K without N increased the nodule number and nodule dry weight as well as the total N in shoots. The applied N fertilizer significantly and linearly reduced the nodule number and fresh and dry weight of nodules. Achakzai 39 found that legume group fertilized with NPK had decreased nodulation and a reduced number of nodules. Vessey 40 demonstrated that the application of N fertilizer to inoculated legumes was unnecessary when good quality inoculant was used. The inoculation method did not influence any of the growth parameters or nodule formation; this because of the effective indigenous rhizo-competitions between microorganisms in the soil and Rhizobium.29 The fertilizer treatments affected the number of nodules, and the nodule dry weight. this finding was s consistent with the results of Ngwu2 who found that the legumes ability to nodulate was affected by NPK treatments at various levels. He also indicated that fertilizer application at various rates affected nodulation and plant height. Ali et al. 38 indicated that P application along with Rhizobium inoculation increased significantly the dry weight of nodules. Similar results were obtained by Javaid.1
Nodule number was significantly enhanced by inoculation with Rhizobium in combination with PK fertilizer. These results differ from those obtained by Umamaheswari et al.41 who found that the number of nodules was not significantly influenced by different fertilizer treatments. The current study indicated that seed inoculation was the most effective method to infect the roots of A. ampliceps and A. salicina seedlings; this result is in accordance with those obtained by Ahmed et al.36 and Huda et al.5 who found that the inoculation of mung bean and Dalbergia sissoo seeds with Rhizobium significantly increased nodulation and plant height. These researches also reported also reported that seed inoculation and N fertilizer significantly increased the number of nodules, the fresh and dry weight of nodules, and the biological yield. Seed, soil, and seed + soil inoculations increased the plant parameters and shoot dry matter.28
Macronutrients accumulation in Acacia species
In both seasons, the N, P and K concentrations (%) in Acacia seedlings varied significantly (p < 0.05) among tree species, fertilizer treatments, and inoculation methods. The average concentration in A. ampliceps seedlings was higher for N, P, and K (%) compared with A. salicina (Figure 1). In A. ampliceps seedlings the Rhizobium treatment gave the highest of N, P concentrations (%) while the PK + Rhizobium had the highest K concentration (%) compared with the control treatment. The NPK + Rhizobium treatment in A. salicina had the highest concentrations of N. P(%) compared with the other treatments (Figure 1). For the inoculation method, the obtained results showed that soil inoculation was associated with higher N, P and K concentrations (%) than seed inoculation across the two growing seasons (Figure 2). A. ampliceps was more responsive to the soil inoculation and had a higher N and K concentration (%) than A. salicina. The P concentration (%) differed between the two inoculation methods in the Acacia species (Figure 2). Generally, the data from the two seasons showed that N, P and K concentrations varied among the tree species with different fertilizer treatments and inoculation methods. Fertilizer has previously been shown to increase foliage N content, particularly in Elaeagnus angustifolia.4,42 Villar-Salvador et al.30 found that seedlings given high rates of fertilizer in the nursery had greater N concentrations than seedlings given low rates of fertilizer either with or without rhizobial inoculation. Also, Oliet et al.43 recommended the application of organic and/or inorganic fertilizers, including P, to nursery-grown saplings of A. salicina to ease the stress of seedling growth. Particularly in nutrient-poor soils in semi or arid environments, such additions increased long-term plantation establishment. These results demonstrated an actual interaction between inoculated rhizobial methods, and host plants in terms growth, competitiveness, and nodulation.
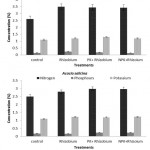 |
Figure 1: The effect of mineral fertilizer treatments on the macronutrients concentration (%) of Acacia species in the two growing seasons
|
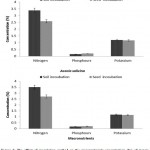 |
Figure 2: The effect of inoculation method on the macronutrients concentration (%) of Acacia species in the two growing seasons
|
Conclusions
The results of this study concluded that inoculation of soil with Rhizobium in combination with fertilizer treatment significantly affected growth and nodulation of Acacia species compared with soil inoculation alone. Seed inoculation with PK + Rhizobium performed better than other treatments and produced the greatest amount of growth and root nodulation. The Rhizobium strain was highly effective on both introduced Acacia seedlings studied here. A. ampliceps was more responsive to inoculation with Rhizobium in conjunction with the fertilizer treatments compared with A. salicina. Concurrent use of fertilizers and inoculation in the nursery, may assist in the production of superior seedlings that may reduce the time needed to achieve canopy closure, thereby helping achieve restoration objectives more rapidly. The study suggested that seed inoculation in conjunction with P and K, but not N, fertilizers will help to accelerate the growth of A. ampliceps and A. salicina in the framework of afforestation and reforestation programs especially in the arid lands of the Riyadh region.
Acknowledgments
The author thanks the College of Food and Agriculture Sciences and the Research Center and the Deanship of Scientific Research, King Saud University, Saudi Arabia and Forestry and Wood Technology Dept., Faculty of Agriculture, Alexandria University for supporting this work.
References
- Javaid A. Growth, no dulation and yield of black gram [Vigna mungo (L.) Hepper] as influenced by biofertilizers and soil amendments. African Journal of Biotechnology. 2009;8:5711.
CrossRef - Ngwu O. E. Effect of NPK 15:15:15 on physicochemical properties, nodulation, and plant height of Arachis hypogea and Voandzeia subterranea using indigenous Rhizobium. Continental Journal of Agronomy. 2007;1:11.
- Khaliq A., Abbasi M. K & Hussain T. Effect of integrated use of organic and inorganic nutrient sources with effective microorganisms (EM) on seed cotton yield in Pakistan. Bioresource Technology. 2006;97:967.
CrossRef - Djumaeva D., Lamers J. P. A., Khamzina A & Vlek P. L. G. The benefits of phosphorus fertilization of trees grown on salinized croplands in the lower reaches of Amu Darya, Uzbekistan. Agroforestry Systems. 2013;87:555.
CrossRef - Huda S. M. S., Sujauddin M., Shafinat S & Uddin M. S. Effects of phosphorus and potassium addition on growth and nodulation of Dalbergia sissoo in the nursery. Journal of Forest Research. 2007;18:279.
CrossRef - Shaben J & Myers J. Relationships between Scotch broom (Cytisus scoparius) soil nutrients and plant diversity in the Garry oak savannah ecosystem. Plant Ecology. 2010;207:81.
CrossRef - Wickens G. E., Seif El Din A .G., Sita G & Nahal I. Role of Acacia species in the rural economy of dry Africa and the Near East. FAO Conservation Guide n. 1995;27. Roma, Italy.
- Aref I. M., Elkhalifah F & El-Juhany L. I. A dendrological key for identification of Acacia species growing in Saudi Arabia and Northern Sudan. Meteorology, Environment and Arid Land Agriculture. Journal of King Abdulaziz University. 2003;14:87.
- Boland D. J., Brooker M. I. H., Chippendale G. M., Hall N., Hyland B. P. M., Johnson R. D., Kleinig D. A., McDonald M.W & Turner J. D. Forest Trees of Australia. CSIRO Publishing, Collingwood, Australia. 2006;768.
- Marcar N. E & Crawford D. F. Trees for saline landscapes. Rural Industries Research and Development Corporation, Canberra, Australia. 2004;246.
- Dommergues Y. R., Duhoux E & Diem H. G. Les arbres fixateurs d’azote caracte´ristiques fondamentales et roˆ le dans l’ame´nagement des e´cosyste`mes me´diterrane´ens et tropicaux. CIRAD, Editions Espaces, FAO, IRD Montpellier. 1999.
- Bontemps C., Rogel M. A., Wiechmann A., Mussabekova A., Moody S., Simon M. F., Moulin L., Elliott G. N., Lacercat-Didier, L., Dasilva C., Grether R., Camargo-Ricalde S. L., Chen W., Sprent J. I., Martínez-Romero E., Young J. P. W & James E. K. Endemic Mimosa species from Mexico prefer alphaproteobacterial rhizobial symbionts. New Phytologist. 2015;10:313.
- Gyaneshwar P., Hirsch A. M., Moulin L., Chen W. M., Elliott G. N., Bontemps C., Estrada-de Los Santos P., Gross E., Reis F. B. D., Sprent J. I., Young J. P & James E. K. Legume-nodulating betaproteo bacteria: diversity host range, and future prospects. Molecular Plant–Microbe Interactions. 2011;24:1276.
CrossRef - Boakye E. Y., Lawson I. Y. D & Danso S. K. A. Characterization and diversity of rhizobia no dulating selected tree legumes in Ghana. Symbiosis. 2016;69:89.
CrossRef - Ludwig F., Dawson T. E., de Kroon H., Berendse F & Prins H. H. T. Hydraulic life in Acacia tortilis trees on East African savanna. Oecologia. 2005;134:293.
CrossRef - Yang L., Liu N., Ren H & Wang J. Facilitation by two exotic Acacia: Acacia auriculiformis and Acacia mangium as nurse plants in South China. Forest Ecology and Management. 2009;257:1786.
CrossRef - Armas C., & Pugnaire F. I. Plant interactions govern population dynamics in a semi-arid plant community. Journal of Ecology. 2005;93:978.
CrossRef - Deaker R., Roughley R. J & Kennedy I. Legume seed inoculation technology a review. Soil Biology and Biotechnology. 2004;36:1275.
CrossRef - Sarr A & Lesueur D. Influence of soil fertility on the rhizobial competitiveness for nodulation of Acacia senegal and Acacia nilotica provenances in nursery and field conditions. World Journal of Microbiology and Biotechnology. 2007;23:705.
CrossRef - Sarr A., Diop B., Peltier R., Neyra M & Lesueur D. Effect of rhizobial inoculation methods and host plant provenances on nodulation and growth of Acacia senegal and Acacia nilotica. New Forests. 2005;29:75.
CrossRef - Giller K. Nitrogen fixation in tropical cropping systems. 2nd ed. CAB International, Wallingford. 2001.
CrossRef - Streeter J. G & Smith R Subba-Rao N. S and Dommergues Y. R. (ed.). Introduction of rhizobia into soils—problems, achievements and prospects for the future In: Microbial interactions in agriculture and forestry Oxford and IBH Publishing, UK. 1998; I.
- Lesueur D & Diouf D., Finan T. M., O’Brian M. R., Layzell D. B., Vessey J. K and W. Newton W.(ed.). Combined effects of rhizobia inoculation and host plant origin on growth and nodulation of Calliandra calothyrsus. In: Nitrogen fixation global perspectives Proceedings of the 13th International Congress on nitrogen fixation Ontario, Canada 27 July CABI Publishing, Wallingford. 2001.
- Vincent J. M. A manual for the practical study of root-nodule bacteria. Blackwell Scientific, Oxford. 1970;1‒166.
- Steel R. G & Torrie J. H. Principles and procedures of statistics. 2nd ed. McGraw Hill, New York. 1980.
- SAS, SAS Institute. The SAS System for Windows CD-ROM for Windows (version 9.13 2008) SAS guide to applications development, 2nd ed., Cary, North Carolina, USA. 2008.
- Ahmadi A. H. A & Chaichi M. R. Nitrogen fertilizing systems and harvest: Frequency effects on percent hard seedless and hard seed breakdown trend in annual medic (Medicago scutellata Var. Robinson). World Journal of Agricultural Sciences. 2007;3:597.
- Bekere W & Hailemariam A. Influences of inoculation methods and phosphorus levels on nitrogen fixation attributes and yield of soybean (Glycine max L.) at Haru, western Ethiopia. American Journal of Plant Nutrition and Fertilization Technology. 2012;2:45.
CrossRef - Dumroese R. K., Jacobs D. F & Davis A. S. Inoculating Acacia koa with Bradyrhizobium and applying fertilizers in the nursery: Effects on nodule formation and seedling growth. HortScience. 2009;44:443.
- Villar-Salvador P., Valladares F., Domı´nguez-Lerena S., Ruiz-Dı´ez B., Ferna´ndez-Pascual M., Delgado A & Pen˜uelas J. L. Functional traits related to seedling performance in the Mediterranean leguminous shrub Retama sphaerocarpa: Insights from a provenance, fertilization, and rhizobial inoculation study. Environmental and Experimental Botany. 2008;64:145.
CrossRef - Shetta N. D. Bio-role of Acacia Karroo in nitrogen fixation at different locations of North-West Egypt region. American-Eurasian Journal of Agricultural & Environmental Sciences. 2010;7:471.
- Sánchez R. C. L., Eichler-Löbermann B., Padilla E. G & Schnug E. Response of Leucaena leucocephala cv. Peru to Rhizobium inoculation under salt stress. Landbauforschung. 2007;4:307.
- Ahmed Z .I., Ansar M., Tariq M & Anjum M. S. Effect of different rhizobium inoculation methods on performance of lentil in Peshawar region. International Journal of Agriculture and Biology. 2008;10:81.
- Molla A. H.,. Shamsuddin Z. H, Halimi M. S., Morziah M & Puteh A. B. Potential for enhancement of root growth and nodulation of soybean co-inoculated with Azospirillum and Bradyrhizobium in laboratory systems. Soil Biology and Biochemistry. 2001;33:457.
CrossRef - Philpotts H. Effect of inoculation method on rhizobium survival and plant nodulation under adverse conditions. Australian journal of experimental agriculture and animal husbandry. 1977;17:308.
CrossRef - Ahmed Z. I., Anjum M. S & Rauf C. A. Effect of rhizobium inoculation on growth and nodule formation of green gram. International Journal of Agriculture and Biology. 2006;8:235.
- Shaheen A & Rahmatullah A. Growth and nodulation of groundnut inoculation with Rhizobium strains based on different carriers. Pakistan Journal of Soil Science. 1996;12:3.
- Ali A., Amjad A., Akhtar J & Yaseen M. Effects of phosphorus in combination with rhizobium inoculation on growth and yield parameters of mungbean. Crop environment. 2010;1:53.
- Achakzai A. K. K. Effect of various levels of nitrogen fertilizer on nodulation of pea cultivars. Pakistan Journal of Botany. 2007;39:1673.
- Vessey J. K. Improvements to the inoculation of pea and lentil crops in Southern Manitoba., ARDI (Agri-Food Research & Development Initiative), Project Results. Manitoba Agriculture, Food and Rural Initiatives, Manitoba, Canada. 2002.
- Umamaheswari P., Padmalatha Y & Rao M. M. Evaluation of different sources of bio-fertilizers in association with inorganic and organic manures in groundnut. Agricultural Science Digest. 2001;21:250.
- Attar H. A., Blavet D., Selim E. M., Abdelhamid M. T & Drevon J. J. Relationship between phosphorus status and nitrogen fixation by common beans (Phaseolus vulgaris L.) under drip irrigation. International Journal of Environmental Science and Technology. 2012;9:1.
CrossRef - Oliet J. A., Planelles R., Artero F & Jacobs D. F. Nursery fertilization and tree shelters affect long-term field response of Acacia salicina (Lindl.) planted in Mediterranean semiarid conditions. Forest Ecology and Management. 2005;215:339.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





