Manuscript accepted on : 22 December 2016
Published online on: --
Plagiarism Check: Yes
In vitro Direct Regeneration of Annona muricata L. from Nodal Explant
N. Abubacker and T. Deepalakshmi
Department of Biotechnology, National College, Tiruchirappalli 620 001, Tamil Nadu, India.
Corresponding Author E-mail: abubacker_nct@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2426
ABSTRACT: Annona muricata L. (Annonaceae) commonly called ‘soursop’ also called prickly custard apple has been used in natural medicine in treating respiratory and digestive ailments. The plant has a poor fruit development and seed setting properties. To bring about proper conservation of this tree, in vitro plantlet regeneration through direct shoot regeneration and rooting protocol was achieved. In vitro callus production was achieved in leaf base explants on MS + BAP 1.5 mg/L+ 2,4-D 2.0 mg/L, callus cell morphogenesis indicated the establishment of proembryo, perfect dichotomy and shoot apices. The axillary shoot formation from nodal explants was achieved on MS + BAP 1.0 mg/L+ Kin 0.5 mg/L+ IBA 1.5 mg/Land rooting in MS + IBA 2.0 mg/L+ NAA 0.5 mg/L+ BAP 1.0 mg/L. The plantlets were acclimatized and thrived in greenhouse and then in natural environmental conditions.
KEYWORDS: Annona muricata L; in vitro culture; morphogenesis
Download this article as:| Copy the following to cite this article: Abubacker M. N, Deepalakshmi T. In vitro Direct Regeneration of Annona muricata L. from Nodal Explant. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Abubacker M. N, Deepalakshmi T. In vitro Direct Regeneration of Annona muricata L. from Nodal Explant. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=21305 |
Introduction
India has a rich and varied heritage of biodiversity, encompassing a wide spectrum of habitats from rain forests to alpine vegetation and from temperate forests to coastal wetlands. In India, about 1500 rare and threatened species of both flowering and non-flowering plant groups were identified during the last two decades. The threat to a majority of them could be attributed to anthropogenic factors like habitat destruction due to grazing, urbanization, other developmental activities and exploitation (Myers, 1988).
A. muricata L. (Annonaceae) commonly called ‘soursop’ also called prickly custard apple, is a small, upright evergreen tree growing 5 to 6 meters in height. Young branchlets are rusty-hairy with malodorous leaves, normally evergreen, alternate, smooth, glossy, dark-green on the upper surface, lighter beneath, oblong, elliptic, pointed at both end, 6-20 cm long and 2-6 cm wide. The flowers are borne singly and may emerge anywhere on the trunk, branches or twigs. They are short stalked, 4-5 cm long, plump and triangular conical; the three fleshy, slightly spreading outer petals are yellow green with three closed set inner petals of pale yellow in colour (De Feo, 1992; Vasquez, 1990) (Fig. 1a-f).
 |
Figure 1 |
The fruit is more or less oval or heart-shaped irregular due to improper carpel development or insect injury. The size ranges from 10-30 cm long and upto 15 cm in width and the weight may be upto 1.5 – 2.5 kg. The fruit is compound and covered with reticulated leathery appearance but tender, inedible bitter skin from which protrude many stubby and curved soft, pliable ‘spines’. The skin is dark-green in the immature fruit, becoming slightly yellowish-green before the mature fruit is soft to touch. Its inner surface is cream coloured mass of fibrous and juicy segments. In aroma, the pulp is somewhat pineapple like, but its musky, subacid to acid. Flovour is unique (Schultes and Raffauf, 1990). Most of the closely packed segments are seedless. In each fertile segment, there is a single oval, smooth, hard, black seed, 1.25–2.00 cm long, and a large fruit may contain upto 200 or more seeds (Morton, 1980) (Fig. 2a-f).
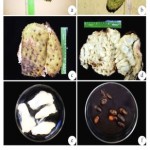 |
Figure 2
|
All parts of the A. muricata tree or used in natural medicine in the tropics including the bark, leaves, root, fruit and seeds. The plant belongs to primitive family Annonaceae and has a long history of use in herbal medicine in the tropical areas in South and North-America including the Amazon and Western Nigeria. But the plant is rarely cultivated in India. The plant requires a lot of attention for conservation and hence an in vitro conservation protocol has been worked out in the present study.
Annona muricata L. is a tropical fruit tree of Annonaceae family which is thought to have originated from the Amazon region where several indigenous varieties are encountered. Despite much research on tissue culture of a wide range of tropical tree species, the Annonaceae has received little attention and only limited success has been reported (Bejoy and Hariharan, 1992). However, the explants had low rooting ability or poor performance during acclimatization. The in vitro propagation protocol has been developed to propagate A. muricata plant through direct regeneration, which will accelerate the process of screening and multiplication of elite trees and facilitate germ-plasm conservation of this medicinal plant. An attempt has been made for in vitro propagation by direct organogenesis methods with an aim that this study might be useful in gaining further information for micropropagation and conservation strategy.
Materials and Methods
Explants Preparation
The third leaf base from the apical portion of axillary branch and the third leaf nodal portion of the plant were used as explants and were tested for culture initiation. The explants were selected during actively growing season before the onset of flowering (August, September and October). These materials were excised from the plants and kept under tap water with Tween-20 (4-5 drops) for 20 min. Leaf base segments (1-2 cm long) was cut from the third young leaves and the nodal portions (1-2 cm long) were treated with fungicide Bavistin (0.5-1.0% w/v) and antibiotic streptomycin (0.5-0.1% w/v) for 10 min each. Each time these explants were rinsed thrice in distilled water and then they were surface sterilized with aqueous solution of HgCl2 (0.1% w/v) for 2-3 min. and washed thrice with sterilized distilled water. The explants were cut gently with a sterilized blade and plants were inoculated in culture tubes with modified MS media under aseptic conditions.
Culture Medium and Culture Conditions
Basal Murashige and Skoog Medium (MS medium, 1962) was prepared using Hi-media and Merck-Chemicals. Sucrose 930% w/v) was added to medium and pH of medium was adjusted to 5.8 before adding agar-agar (8% w/v). Different growth regulators 6-Benzylaminopurine (BAP), 2,4-Dichlorophenoxy acetic acid (2,4-D), 6-Furfurylaminopurine (Kinetin-kin), Indole 3-acetic acid (IAA), Indole 3-butyric acid (IBA), a-Naphthaleneacetic acid (NAA) at different concentrations and combinations were added to medium. In the present study, the medium were autoclaved at 121°C and 15 lbs pressure for 20 min. All the in vitro cultures were maintained at 24 ± 2°C and illuminated for 16 h with fluorescent light (18-24 mmol/m/sec) followed by 8 h dark period and the relative humidity was about 60-80% within the 25´150 mm culture tubes covered with cotton plug.
Callus Induction
The healthy and normal leaf base segment of the third leaf was inoculated in MS medium with different combinations of growth regulators. Twenty-five cultures were raised for each treatment and all experiments. BAP, 2,4-D and IBA combinations were tested to estimate the callus production (Fig. 3a-f).
 |
Figure 3
|
Callus Cell Morphogenesis
The morphogenesis of callus cell of A. muricata was conducted by using the following protocol (Siew-Hwa Kwa et al., 1997). Aseptically grown callus were cut into five pieces using a scalpel. These were cultured on liquid MS medium supplemented with 2% (w/v) sucrose and 0.5 mg/L2.4-D in 100 ml Erlenmeyer flasks. The flasks were capped with two layers of aluminium foil and sealed with a layer of parafilm ‘M’ (Pechiney Chicago, IL60631). Cultures were agitated at 150 rpm on a rotary shaker. All cultures were incubated under continuous illumination using cool-white fluorescent lamps. Increase in fresh weights and settled cells, volume of the cells in culture were followed at three to four-day intervals for a period of two weeks. The sub-cultured cell suspension cultures which consisted of 1 ml settled cells from the suspension culture. 0.1 ml culture was used for cell morphogenesis study. To this a drop of safranin stain was added and the slides were prepared, viewed and photographed with 400x magnifications (Fig. 4a-f).
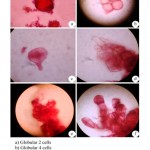 |
Figure 4
|
Axillary shoot induction
The healthy nodal segments were inoculated in MS medium with different combinations of growth regulators. Twenty-five cultures were raised for each treatment, BAP, kinetin, IAA and IBA combinations were tested for shoot induction (Fig. 5a-f).
 |
Figure 5
|
Root induction
The axiliary shoots were subjected to root induction in MS medium with different combinations of growth regulators. Ten cultures were raised for each formulation, IAA, IBA, NAA and BAP combinations were tested for root induction (Fig. 6a-b). The rooted shoot were transferred to mist-chamber and then to green-house condition (Fig. 6c-f).
 |
Figure 6
|
Results
Callus Induction
The combination of BAP, 2,4-D and IBA induced an excellent amount of callus from leaf base explant of Annona muricata and the morphology of callus was yellowish green and greenish in colour and friable in its nature. The various combination of growth regulators used for this study for callus induction is given in (Table-1).
Table 1: Callus induction from leaf base explants of A. muricata cultured on MS medium supplemented with various concentrations and combination of growth regulators
| Concentration and combination of plant growth regulators | No. of explants
inoculated |
No. of explants forming
callus |
Morphology
of callus |
| MS + BAP 0.5 mg/L+ 2,4-D 0.5 mg/L | 25 | 4 | G, F |
| MS + BAP 1.0 mg/L+ 2,4-D 0.5 mg/L | 25 | 4 | YG, G, F |
| MS + BAP 1.5 mg/L+ 2,4-D 0.5 mg/L | 25 | 6 | YG, G, F |
| MS + BAP 2.0 mg/L+ 2,4-D 0.5 mg/L | 25 | 6 | YG, G, F |
| MS + BAP 2.5 mg/L+ 2,4-D 0.5 mg/L | 25 | 4 | G, F |
| MS + BAP 0.5 mg/L+ 2,4-D 1.0 mg/L | 25 | 6 | YG, G, F |
| MS + BAP 1.0 mg/L+ 2,4-D 1.5 mg/L | 25 | 6 | YG, G, F |
| MS + BAP 1.5 mg/L+ 2,4-D 2.0 mg/L | 25 | 8 | YG, G, F |
| MS + BAP 2.0 mg/L+ 2,4-D 2.5 mg/L | 25 | 6 | YG, G, F |
| MS + BAP 2.5 mg/L+ 2,4-D 3.0 mg/L | 25 | 4 | YG, G, F |
| MS + IBA 0.5 mg/L+ 2,4-D 0.5 mg/L | 25 | 4 | G, F |
| MS + IBA 1.0 mg/L+ 2,4-D 0.5 mg/L | 25 | 4 | G, F |
| MS + IBA 1.5 mg/L+ 2,4-D 0.5 mg/L | 25 | 4 | G, F |
| MS + IBA 2.0 mg/L+ 2,4-D 0.5 mg/L | 25 | 4 | G, F |
| MS + IBA 2.5 mg/L+ 2,4-D 0.5 mg/L | 25 | 4 | G, F |
F = Friable; G = Green; YG = Yellowish Green
The healthy leaf base segment as explants were inoculated in MS medium with different combinations of growth regulators. Twenty-five cultures were raised for each treatment. MS+BAP 1.5 mg/L+ 2,4-D 2.0 mg/Lproduced the maximum calli induction in eight culture tubes followed by MS+BAP 1.5, 2.0 mg/L+ 2,4-D 0.5 mg/Lalso MS+BAP 1.5, 1.0 and 2.0 mg/L+ 2,4-D 1.0, 1.5 and 2.5 mg/Lproduced calli in six culture tubes respectively. The other growth regulator combination produced less number of calli (Fig. 1a-f).
Callus cell Morphogenesis of Annona Muricata
The capacity of cultured plant tissues and callus cells to undergo morphogenesis resulting in formation of discrete organs or whole plants. Callus cell morphogenesis conducted using callus cell culture in MS liquid medium supplemented with BAP 1.0 mg/L+ Kin 0.5 mg/L+ IBA 1.5 mg/L combination produced maximum callus induction. The cell showed varied morphology from globular, ovoid to elliptical form and some cells were transformed into proembryo. The later stage of morphogenesis produced establishment of apical cells, perfect dichotomy and establishment of shoot apices (Fig. 2a-f).
Axillary shoot formation
MS medium supplemented with BAP, Kin, IAA and IBA responded variable effects on the axillary shoot formation in nodal explants. The maximum number of 12 shoots was recorded on MS + BAP 1.0 mg/L+ Kin 0.5 mg/L+ IBA 1.5 mg/L followed by 10 shoots was recorded on MS + BAP 0.5 mg/L+ Kin 0.5 mg/L+ IBA 2.0 mg/Lout of 25 explants inoculated. The other combinations showed lesser number of shoot formation and MS + BAP 0.5 mg/L+ Kin 0.5 mg/L+ IAA 0.5 mg/L or IAA 1.0 mg/L failed to produce shoots (Table 2 and Fig. 3a-f).
Root Induction
The different concentration and combination of IAA, NAA, BAP and IBA showed variations in root formation. The maximum number of 8 explants out of 10 produced roots in MS + IBA 2.0 mg/L+ NAA 0.5 mg/L+ BAP 1.0 mg/L followed by 6 explant in MS + IBA 2.0 mg/L+ NAA 0.5 mg/L+ BAP 0.5 mg/Las well as MS + IBA 2.0 mg/L+ NAA 0.5 mg/L+ BAP 1.5 mg/L. The other combinations showed lesser number of explants forming root and MS + IAA 0.5 mg/L or 1.0 mg/L+ NAA 0.5 mg/L+ BAP 0.5 mg/L failed to produce roots (Table 3 and Fig. 4a, b).
Table 2: Axillary shoot formation from nodal explants of A. muricata cultured on MS medium supplemented with various combination and concentrations of growth regulators
| Concentration and combination of plant growth regulators | No. of explants
inoculated |
No. of explants forming axillary shoot |
| MS + BAP 0.5 mg/L+ Kin 0.5 mg/L+ IAA 0.5 mg/L | 25 | NIL |
| MS + BAP 0.5 mg/L+ Kin 0.5 mg/L+ IAA 1.0 mg/L | 25 | NIL |
| MS + BAP 0.5 mg/L+ Kin 0.5 mg/L+ IAA 1.5 mg/L | 25 | 6 |
| MS + BAP 0.5 mg/L+ Kin 0.5 mg/L+ IAA 2.0 mg/L | 25 | 10 |
| MS + BAP 0.5 mg/L+ Kin 0.5 mg/L+ IAA 2.5 mg/L | 25 | 4 |
| MS + BAP 1.0 mg/L+ Kin 0.5 mg/L+ IBA 0.5 mg/L | 25 | 2 |
| MS + BAP 1.0 mg/L+ Kin 0.5 mg/L+ IBA 1.0 mg/L | 25 | 6 |
| MS + BAP 1.0 mg/L+ Kin 0.5 mg/L+ IBA 1.5 mg/L | 25 | 12 |
| MS + BAP 1.0 mg/L+ Kin 0.5 mg/L+ IBA 2.0 mg/L | 25 | 6 |
| MS + BAP 1.0 mg/L+ Kin 0.5 mg/L+ IBA 2.5 mg/L | 25 | 4 |
| MS + BAP 1.5 mg/L+ Kin 0.5 mg/L+ IBA 0.5 mg/L | 25 | 4 |
| MS + BAP 1.5 mg/L+ Kin 1.0 mg/L+ IBA 0.5 mg/L | 25 | 4 |
| MS + BAP 1.5 mg/L+ Kin 1.5 mg/L+ IBA 0.5 mg/L | 25 | 4 |
| MS + BAP 1.5 mg/L+ Kin 2.0 mg/L+ IBA 0.5 mg/L | 25 | 6 |
| MS + BAP 1.5 mg/L+ Kin 2.5 mg/L+ IBA 0.5 mg/L | 25 | 2 |
Table 3: Root induction from nodal explants of A. muricata cultured on MS medium supplemented with various combination and concentrations of growth regulators
| Concentration and combination of plant growth regulators | No. of explants
inoculated |
No. of explants forming roots |
| MS + IAA 0.5 mg/L+ NAA 0.5 mg/L+ BAP 0.5 mg/L | 10 | NIL |
| MS + IAA 1.0 mg/L+ NAA 0.5 mg/L+ BAP 0.5 mg/L | 10 | NIL |
| MS + IAA 1.5 mg/L+ NAA 0.5 mg/L+ BAP 0.5 mg/L | 10 | 2 |
| MS + IAA 2.0 mg/L+ NAA 0.5 mg/L+ BAP 0.5 mg/L | 10 | 2 |
| MS + IAA 2.5 mg/L+ NAA 0.5 mg/L+ BAP 0.5 mg/L | 10 | 2 |
| MS + IBA 0.5 mg/L+ NAA 0.5 mg/L+ BAP 0.5 mg/L | 10 | 2 |
| MS + IBA 1.0 mg/L+ NAA 0.5 mg/L+ BAP 0.5 mg/L | 10 | 2 |
| MS + IBA 1.5 mg/L+ NAA 0.5 mg/L+ BAP 0.5 mg/L | 10 | 4 |
| MS + IBA 2.0 mg/L+ NAA 0.5 mg/L+ BAP 0.5 mg/L | 10 | 6 |
| MS + IBA 2.5 mg/L+ NAA 0.5 mg/L+ BAP 0.5 mg/L | 10 | 2 |
| MS + IBA 2.0 mg/L+ NAA 0.5 mg/L+ BAP 0.5 mg/L | 10 | 2 |
| MS + IBA 2.0 mg/L+ NAA 0.5 mg/L+ BAP 1.0 mg/L | 10 | 8 |
| MS + IBA 2.0 mg/L+ NAA 0.5 mg/L+ BAP 1.5 mg/L | 10 | 6 |
| MS + IBA 2.0 mg/L+ NAA 0.5 mg/L+ BAP 2.0 mg/L | 10 | 4 |
| MS + IBA 2.0 mg/L+ NAA 0.5 mg/L+ BAP 2.5 mg/L | 10 | 2 |
Acclimatization
The rooted axillary shoots were transferred to small pots containing autoclaved sand. Those pots were covered initially with polythene bags to maintain humidity and placed in mist chamber. Every alternate day, quarter strength of MS medium salt solution was supplied to the plantlets. After two weeks of growth, the complete plants were established, acclimatized and thrived in greenhouse condition and then in natural environmental conditions (Fig. 4 c-f).
Discussion
The maximum number of eight calli were produced in MS + BAP 1.5 mg/L+ 2,4-D 2.0 mg/Lin A. muricata leaf base explants. The other combinations produced lesser number of calli in a total of twenty-five tubes inoculated. Li-Da Wang et al. (2002) obtained callus cultured on Gamborg B5 medium + NAA 5.0 mg/L+ 2-catin 4.0 mg/L. There was no report on in vitro callus production of A. muricata. Many reports were available in in vitro callus production from other genera. However the role of BAP and 2,4-D is always to induce calli, but there will be variations from plant to plant. This is to be stated that the callus induction from leaf base explants in A. muricata is the first report of its kind.
Callus Cell Morphogenesis
The capacity of cultured plant callus cells to undergo morphogenesis resulting in the formation of discrete organs or whole plants has provided opportunities for numerous application of in vitro plant biology. The cell showed varied morphology from globular, ovoid to elliptical cells. Some cells were transformed into pro-embryo. The later stage of morphogenesis resulted perfect dicotomy and establishment of shoot apex. The in vitro morphogenesis of plant cells would lead to the genetic bases of cell transformation study and stimulate progress in functional genomic research as stated by Phillips (2004).
Axillary shoot formation
Axillary shoot formation in vitro was achieved in maximum number of 12 shoot on MS + BAP 1.0 mg/L+ Kin 0.5 mg/L+ IBA 1.5 mg/L followed by 10 shoots on MS + BAP 0.5 mg/L +Kin 0.5 mg/L+ IAA 2.0 mg/Lout of 25 explants inoculated. Lemos and Baker (1998) used internodal explants of A. muricata to induce shoot regeneration on Nitsch media. The highest percentage (7%) of explants produced shoots in sorbitol 10-30 g/Las carbon source supplemented with BAP 2.0 mg/L+ NAA 0.5 mg/L. Zobayed et al. (2001) investigated A. muricata, nodal explants and produced multiple shoots in MS + BA 1.0 mg/L+ NAA 0.1 mg/L. Done Dipre et al. (2012) produced multiple direct organogenesis in A. muricata from immature hypocotyl explants in MS + BAP 2.25 mm + IBA 2.5 mm concentration. Bejoy and Hariharan (1992) used half strength MS medium with 20 g/L sucrose and 7 g/L agar + BA (6 benzyladenine) 2.2-13.3 mM + NAA 0.27-2.7 mm concentration for shoot induction. The present report shows variation in shoot formation, probably due to the medium used was MS and carbon source was sucrose, since the role of Kin and IBA are vital along with BAP in shoot regeneration.
Root Induction
The two week old regenerated shoots were transferred to different combinations of rooting medium. The maximum number of 8 explants out of 10 produced roots in MS + IBA 2.0 mg/L+ NAA 0.5 mg/L+ BAP 1.0 mg/L. The combinations of BAP + IBA induce rooting in many plant species. Bejoy and Hariharan (1992) induced roots in A. muricata on half strength MS medium with 20 g/l, sucrose 7 g/L+ IBA 4.9-14.8 mM concentration. The root induction in A. muricata with combination of IBA, NAA and BAP is a new report of this kind.
Conclusion
The in vitro protocol for direct plantlet regeneration from internodal explants of A. muricata will be useful in gaining more information for micropropagation technique of this rare tree member for conservation strategy.
Acknowledgements
Authors expresses thanks to Padmavibhushan Dr. V. Krishnamurthy, President, Sri. K. Ragunathan, Secretary and Dr. K. Anburasu, Principal, National College, Tiruchirappalli for all the supports and encouragement given to PG and Research Department of Biotechnology to carry over the research work.
References
- Bejoy M and Hariharan M. In vitro plantlet differentiation in Annona muricata. Plant Cell Tissue and Organ Culture. 1992;31:245-247.
- Feo V. D. Medicinal and magical plants in the Northern Peruvian Andes. Fitoterapin. 1992;63:417-440.
- Dipre D., Chavarria Y., Mejia A., Castillo M. B and Vega W. L. Multiple direct organogenesis in sorusop (Annona muricata L.). Journal of Biotechnology. 2012;1-10.
- Lemos E. E. P and Baker D. A. Shoot regeneration in response to carbon source on intermodal explants of Annona muricata L. Plant Growth Regulation. 1998;25:105-112.
CrossRef - Li-Da W., De-You Q., Ji-Yun C., Yi-Fan H., Jun-Hua Z and De-An G. Callus cultures of Annona squamosa for the production of Annonaceous acetogenins. Journal of Asian Natural Products Research. 2002;4:171-174.
CrossRef - Morton J. F. Caribbean and Latin American Folk Medicine and its influence in the United States, Q. J. Crude Drug Res. 1980;18:57-75.
CrossRef - Murashige T and Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962;15:473-493.
CrossRef - Myers N. Threatened biotas ‘hot spots’ in tropical forests. Environmentalist. 1988;8:187-208.
CrossRef - Philips G. C. In vitro morphogenesis in plant recent advances. In: Goodman, R. M. (ed.). Encyclopedia of Plant and Crop Sciences, New York. 2004;2:579-583.
CrossRef - Schultes R. E and Raffauf A. The Healing Forest: Medicinal and Toxic Plants of the Northwest Amazonia Historical, Ethno and Economic Botany Series. 1990;2. Dioscorides Press, Portland.
- Siew-Hwa K., Yeow-Chin W., Tit-Meng L and Kumar P. P. Morphogenetic plasticity of callus reinitiated from cell suspension cultures of the fern Platycerium coronarium. Plant Cell Tissue and Organ Culture. 1997;48:37-44.
CrossRef - Vasquez M. R. Useful Plants of Amazonian Peru. Second Draft. Filed with USDA’s National Agricultural Library. USA. 1990.
- Zobayed S. M. A., Armstrong J and Armstrong W. Multiple shoot induction and leaf and flower bud abscission of Annona cultures as affected by types of ventilation. Plant Cell Tissue and Organ Culture. 2001;69:155-165.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





