Manuscript accepted on : 25 February 2017
Published online on: --
Plagiarism Check: Yes
Nahid Rahimifard1, Leila Moslemi2, Nooshin Aghilee3 and Mandana Moghni4
1Department of Microbiology, Food and Drug Control Laboratories(FDCL), Minstry of health and medical Education (MOH), Tehran,Iran. Islamic Azad University, Pharmaceutical Sciences Branch, Faculty of Pharmacy, Tehran, Iran.
2Islamic Azad University, Pharmaceutical Sciences Branch, Faculty of Pharmacy, Tehran, Iran.
3Department of Food and Water Borne Disease. Tehran, Iran.
4Sharecord University of medical sciences, Iran.
Corresponding Authors E-mail: leila.moslemi1@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2438
ABSTRACT: Dionysia revoluta Boiss is a member of primulaceae.This plant is distributed locally across south region of Iran. Antimicrobial activities against some species have been reported from the extracts and fractions of this plant. In the present study antimicrobial activities of the methanol , chloroform and crude extracts against 50 isolates of Acinetobacter baumannii from wound of burned patients hospitalized at Motahari hospital in Tehran and Acinetobacter baumannii ATCC 10572 were evaluated. The samples of plant were collected from Hormozgan province. The plant were first dried and blended and extracts were prepared by standard methods of maceration. The extracts then were allowed to air dried. The dried concentrated extracts were kept within glass vials under standard conditions until used. 50 isolates of Acinetobacter baumannii from wound of burned patients were diagnosed by GNA-GNB microgen kit. Acinetobacter baumannii ATCC 10572 was used as standard strain. Standard agar diffusion methods (disk diffusion and well diffusion) were used to examine the antimicrobial effects of different concentrations of plant extracts against the bacteria. All three extracts had antibacterial effects on Acinetobacter baumannii. Water extract at 2000µg/ml showed the maximum inhibition zone (15 mm). Well diffusion methods was more efficient method and sixteen isolates (32%) were shown growth inhibition zone to all extracts and showed growth inhibition zone from 12 mm to 15 mm in this method. We concluded that the antimicrobial activity of Dionysia revoluta Boiss against clinical isolates of Acinetobacter baumannii is valuable. Further investigation for determining of MIC and MBC and especially in vivo studies are recommended.
KEYWORDS: Acinetobacter baumannii; Antimicrobial burned patients; Dionysia revoluta Boiss;
Download this article as:| Copy the following to cite this article: Rahimifard N, Moslemi L, Aghilee N, Moghni M. Antibacterial Effect of Dionysia Revoluta Boiss.Extracts on Acinetobacter Bumannii Isolated from Wound of Burned Patients. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Rahimifard N, Moslemi L, Aghilee N, Moghni M. Antibacterial Effect of Dionysia Revoluta Boiss.Extracts on Acinetobacter Bumannii Isolated from Wound of Burned Patients. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=22343 |
Introduction
Flowers and plants are the most telling symbol of power and greatness of the creation. The value of this gift from God, not only in human and animal food supply But the cure and relieve pain in many human person can be found in plants. Study and research on drugs that are vegetable or animal origin is done in the field of Pharmacognosy and Pharmacology .This process of evolution today is one of the specific fields of pharmacy education in several main branches. Although the vast majority of drugs are chemical but it’s estimated that at least a third of all pharmaceutical products are from herbal sources or after extraction of plants are modified.Biotechnology tries to produce new group of biological medicines and introduces a new drug design methods.Use of medicinal plants to treat diseases has a long history and now also in many developed countries is a main way for treatments.Iran has long been used in herbal remedies so that in ancient Iranian medical resources such as the writings of Avicenna (Ibn Sina) many sections devoted to this topic and plant diversity, with almost 9500 species of vascular plants in Iran is more than the entire continent of Europe.
There are a huge number of researches about antimicrobial effects of Iranian plants derivatives(Aminnezhad et al., 2012; Hajimehdipoor et al., 2010; Rahimifard et al., 2012- Rahimifard et al., 2014; Rahimifard et al., 2008; Rahimifard et al., 2015; Beiki et al., 2016; Amiri et al., 2016; Hafezan et al., 2016; Safarian et al., 2016; Mehrara et al., 2014). Due to microbial resistance against antibiotics and side effects of chemical drugs, it is necessary to obtain new anti-bacterial compounds. Acinetobacter baumannii is one of the important pathogens in this respects.( Fournier et al.2006; Kim et al.,2004; Antunes et al., 2011)
Antimicrobial activities against some species have been reported from the extracts and fraction of Dionysia revoluta Boiss( Ahani et al., 2016;Mashhadi et al., 2016; Rahimifard et al., 2016). In the present study antimicrobial activities of methanolic and chloroformic and crude extracts against 50 clinical isolates of Acinetobacter baumannii from the wound of burned patients hospitalized at Motahari hospital in Tehran and Acinetobacter baumannii ATCC 10572 were evaluated.
Methods and Materials
Plant Material and Preparation of Extracts
The samples of plant were collected from Hormozgan province of Iran. The plant were dried with rotary evaporator, blended and extracts were prepared by standard methods of maceration. Methanolic extract of Dionysia revoluta Boiss were extracted with methanol 80%(1:10) by using maceration method for 4 days. After every 24 h, the mixture was filtered and new solvent was added to the plant powder. The extract were concentrated under reduced pressure to dryness and 8 concentrations of methanol, chloroform and water extract of Dionysia revoluta ( 2000, 1000, 500, 250, 62.5,125, 31.25, 15.6 μg / ml) were prepared.
Bacterial Isolation and Identification
In the present study 50 clinical isolates of Acinetobacter baumannii were used. All clinical strains were isolated from the Burning wound of patients hospitalized at Motahari hospital in Tehran. Primary isolation was performed on EMB(Merck) and also Blood agar base plates supplemented with 7% ship blood, after incubation at 37°C, with aerobic atmosphere , for 24 hours. Following primary selective isolation, Acinetobacter baumannii samples were identified by usual diagnostic procedures, i.e. according to colony morphology, gram staining and biochemical tests and Microgen identification kit GN A and GN B.(Microgen Bioproducts)
Disk Diffusion Agar Method
Agar disk diffusion assay (Cup plate assay)was carried out on Muller-Hinton agar(Merck1.05437.0500) by Kirby-Bauer disk diffusion susceptibility test protocol. Muller-Hinton agar was poured in sterile plates and plate’s surfaces were inoculated with approximately (1.5*108 CFU/ml) equal to 0.5 McFarland turbidity of inoculum of 50 clinical isolates of Acinetobacter baumannii and Acinetobacter baumannii ATCC 10572 as Standard strain by sterile swab. The inoculum optical density (OD) had been adjusted between 0.08-0.13 in 620 nm in spectrophotometer. Standard blank disk with 6.4 mm diameter were put on plate (with an approximate distance of 19 mm . (Shokraei et al., 2014) 8 concentrations of methanol, chloroform and water extract of Dionysia revoluta ( 2000, 1000, 500, 250, 62.5,125, 31.25, 15.6 μg / ml) were prepared and 20 µl of each was poured on each blank disk and plates were incubated for 24 hours at 37°C with closed lid and aerobe conditions. Then the diameters of absence of growth were measured.
Well Diffusion Agar Method
Agar well diffusion assay was carried out on Muller-Hinton agar. Muller-Hinton agar (Merck1.05437.0500) was poured in sterile plates and plate’s surfaces were inoculated with approximately (1.5*108 CFU/ ml) equal to 0.5 McFarland turbidity of Acinetobacter baumannii strains by a sterile swab. The inoculum optical density (OD) had been adjusted between 0.08-0.13 in 620 nm in spectrophotometer. Wells were cut on plate by sterile Pasteur pipet (with an approximate distance of 19 mm). Wells were filled by 100 µl of 8 concentrations of methanol, chloroform and water extract of Dionysia revoluta ( 2000, 1000, 500, 250, 62.5,125, 31.25, 15.6 μg / ml) and plate were incubated for 24 hours at 37°C with closed lid and aerobe conditions. The clear zone around wells then was recorded. (Barzavar et al., 2015)
Results
Table 1: Average inhibition zone diameter in millimeters in three times for different concentrations of methanol, chloroform and aqueous extracts of Dionysia revoluta on 16 of 50 strains of Acinetobacter baumannii in disc method (discs diameter are 7 mm.)
| concentration
µg/ml extract |
2000 | 1000 | 500 | 250 | 125 | 62.5 | 31.25 | 15.6 | Gentamycin
10µg |
|||||
| Methanol extract | 14.0±0.5 | 12.7±0.5 | 10.7±0.5 | 9.2±0.0 | 8.3±0.0 | 7.5±0.0 | NIZ | NIZ | ||||||
| Chloroform extract | 13±0.5 | 12.0±0.5 | 10.2±0.5 | 8.6±0.0 | 7.2±0.0 | 7.0±0.5 | NIZ | NIZ | ||||||
| Crude extract | 15±5.0 | 14.3±0.7 | 13.7±0.5 | 12.9±0.3 | 11.7±0.5 | 10.7±0.5 | NIZ | NIZ | ||||||
NIZ: No Inhibition Zone/Resistant
According to the table all the plant extracts in concentrations less than 62.5 micrograms per milliliter has no effect on the growth of A. baumannii (50 clinical strains).
In general, the smallest diameter of inhibition was in extracts of methanol and chloroform fraction in a concentration of 62.5 micrograms per milliliter. And the aqueous fraction at a concentration of 2000 micrograms per milliliter has maximum inhibition zone which is equal to 15 mm.
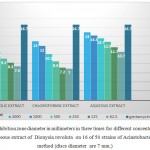 |
Graph 1: Average inhibition zone diameter in millimeters in three times for different concentrations of methanol, chloroform and aqueous extract of Dionysia revoluta on 16 of 50 strains of Acinetobacter baumannii in disc method (discs diameter are 7 mm.)
|
Table 2: Average inhibition zone diameter in millimeters in three times for different concentrations of methanol, chloroform and water extract of Dionysia revoluta on 16 of the 50 strains of Acinetobacter baumannii in well method.
| concentration
µg/ml extract |
2000 | 1000 | 500 | 250 | 125 | 62.5 | 31.25 | 15.6 | Gentamycin
10µg |
| Methanol extract | 15.0±0.5 | 14.14±0.7 | 13.0±0.0 | 12.3±0.7 | 11.3±0 | NIZ | NIZ | NIZ | 25 |
| Chloroform extract | 14.0±0.3 | 13.7±0.5 | 12.3±0.7 | 11.0±0 | 10.2±0.5 | NIZ | NIZ | NIZ | |
| Crude extract | 16.0±1.0 | 15.0±1.0 | 14.7±0.5 | 12.7±0.5 | 11.9±0.5 | 10.7±0.5 | NIZ | NIZ |
NIZ: No Inhibition Zone.Resistant
According to the table all the plant extracts in concentrations less than 62.5 micrograms per milliliter has no effect on Acinetobacter baumannii (50 clinical strains).
The smallest inhibition zone was for chloroform fraction in a concentration of 125 micrograms in diameter ml (equal to 2.10 mm). And the aqueous fraction at a concentration of 2000 micrograms per milliliter had the greatest inhibition which is equal to to 16 mm.
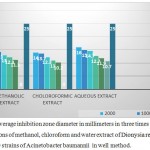 |
Graph 2: Average inhibition zone diameter in millimeters in three times for different concentrations of methanol, chloroform and water extract of Dionysia revoluta on 16 of the 50 strains of Acinetobacter baumannii in well method.
|
Table 3: Average inhibition zone diameter in millimeters in three times for the effect of different concentrations of methanol, chloroform and aqueous fractions extracts of Dionysia revoluta on the strains of Acinetobacter baumannii ATCC 10572 in disc method (discs diameter are7 mm).
| concentration
µg/ml extract |
2000 | 1000 | 500 | 250 | 125 | 62.5 | 31.25 | 15.6 | Gentamycin
10µg |
| Methanol extract | 19.0±0.5 | 18.5±0.5 | 17.7±0.5 | 16.3±0.7 | 16.0±0.5 | NIZ | NIZ | NIZ | 25 |
| Chloroform extract | 18.0±0.5 | 16.7±0.3 | 15.7±0.3 | 14.7±0.5 | 13.7±0.5 | NIZ | NIZ | NIZ | |
| Crude extract | 5.0± 20 | 18.7±0.5 | 17.3±0.7 | 16.7±0.3 | 16.7±0 | 15.7±0.5 | 13.3±0.7 | NIZ |
NIZ: No Inhibition Zone.Resistant
According to table all the plant extracts in concentrations of less than 31.25 micrograms per milliliter has no effect on the growth of standard A. baumannii strains ATCC 10572.
The smallest diameter of inhibition in the aqueous fraction at concentrations 31.25 micrograms per milliliter (3.13 mm) and then in chloroform fractions at concentration of 125 micrograms per milliliter (7.13 mm) were observed and the aqueous fraction in a concentration 2000 micrograms per milliliter had the most inhibition zone (20 mm).
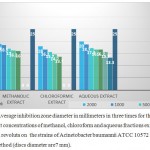 |
Graph 3: Average inhibition zone diameter in millimeters in three times for the effect of different concentrations of methanol, chloroform and aqueous fractions extracts of Dionysia revoluta on the strains of Acinetobacter baumannii ATCC 10572 as standard in disc method (discs diameter are7 mm).
|
It should be noted that the results in the well method on A. baumannii ATCC 10572 as standard were also quite similar results were obtained in a disk method and as well as a negative control for each of the extracts of methanol, water and chloroform fractions,in the entire process was ineffective.
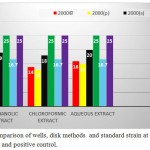 |
Graph 4: Comparison of wells, disk methods and standard strain at concentration 2000 μg . ml and positive control.
|
All three extracts had antibacterial effects on Acinetobacter baumannii.Water extract at 2000 mg/ml showed the maximum inhibition zone (15 mm).Well diffusion methods was more efficient method. Sixteen isolates were sensitive to all extracts and showed growth inhibition zone from 12 mm to 15 mm. We concluded that the antimicrobial activity of Dionysia revoluta Boiss against Acinetobacter baumannii is valuable, but further investigation especially in vivo studies are recommended before final conclusion.
Suggestions
As you know, historically, the most important infectious diseases are the main causes of death in humans. Today, many synthetic drugs are manufactured with antibacterial properties. Although these drugs can effect on micro organism, they have side effects for human. In addition, due to the indiscriminate use of chemical agents resistance to antimicrobial agents has been created. The use of plants with medicinal properties so recently expanded to deal with the bacteria. Although antibacterial and antifungal effects of plants that have been studied are too high, Still, there are plants among the plants that have long been used as a medicinal substance but their biological effects have not been studied yet. In the meantime, Primrose families like Dionysia revoluta due to their antibacterial effects have been demonstrated in recent years are given priority.In vivo tests subsequently recommended in order supplying pharmaceutical products that can be used in the treatment of diseases.It is suggested to separate and purification the antibacterial and antifungal compounds in plant extracts and fractions that the main factor responsible for the antimicrobial activity be detected So maybe we can change the molecular structure of this compound with an effective antimicrobial products introduced. It is hoped that in future more research on the antimicrobial effects of this plant have been conducted on different bacterial species and by determining the antimicrobial active ingredients of the plant, separation, purification, formulation and preparation of various dosage forms of that, is an excellent step for illnesses that are created by different bacterial species to be removed.
Acknowledgement
The author sincerely thanks the Sarv Saadat Laboratory complexes in West sarv, Saadat abad, Tehran for their financial support and kind assistant and hard efforts.
References
- Aminnezhad S., Rahimifard N., Kermanshahi R. K., Ranjbar R., Baghery O., Bagheri F. Synergistic effects of supernatant from Lactobacillus plantarum cultured medium and conventional antibiotics against Pseudomonas aeruginosa. Biosciences Biotechnology Research Asia. 2012;9(2):457-466.
CrossRef - Hajimehdipoor H., Samadi N., Mozaffarian V., Rahimifard N., Shoeibi S.,Hamedani M. P. Chemical composition and antimicrobial activity of Oliveria decumbens volatile oil from west of Iran . Journal of Medicinal Plants. 2010;9(6):39-44.
- Rahimifard N., Sabzevari O.,Shoeibi S., Pakzad S. R., Ajdari S. Antifungal activity of the essential oil of Thymus vulgaris on Candida albicans, Aspergillus niger and Aspergillus flavus. Journal of Pure and Applied Microbiology. 2008;2(2):343-346.
- Rahimifard N., Bagheri E., Asgarpanah J.,balajadeh K. B. Study of the Antibacterial Activity of Total Extract and Petroleum Ether, Chloroform, Ethyl Acetate and Aqueous Fractions of Aerial Parts of Heliotropium bacciferum against Staphylococcus aureus, Bacillus cereus, Pseudomonas aeruginosa, E. coli, Salmonella enteritidis .Biosciences Biotechnology Research Asia. 2014;11(1):239-2485.
- Rahimifard N., Rabiei M.,Beitolahi L., Ahi K. Helicobacter pylori and the herbal compound effect. Biosciences Biotechnology Research Asia. 2012;7(2):647-649. 6.
- Beyki S., Rahimifard N., Daryani N. E ., Khaniki G. B., Rostamzadeh H. Study on antibacterial activity of Juniperus exelca, Juniperus communis, Juniprus samata extract from native region of Semnan province on Helicobacter pylori and compare their effects. Iranian Journal of Public Health. 2016;45:39 7.
- Amiri E., Rahimifard N.,Janat B.,Shoeibi S., N Amiri S. Study of antifungal activity of aqueous and hydroalcoholic extracts of Portltlaca oleracea L. on Aspergillus flavus, Aspergillus niger and Candida albicans. Iranian Journal of Public Health. 2016;45:60 8.
- Safarian F., Rahimifard N.,Shojaii A. Antifungal activity of methanol, hexane, chloroform, ethyl acetate and aqueous fractions of the aerial parts of Euphorbia esula L. on Candida albicans, Candida krusei, Aspergillus niger and Aspergillus fumigatus. Iranian Journal of Public Health. 2016;45:71.
- Mashhadi N., Rahimifard N., Motamed S. M.,Pourakbari B. Antifungal activity of aqueous, methanol and chloroform, extracts of Dionysia revoluta belonging to Priniulaceae family. Iranian Journal of Public Health. 2016;45:51.
- Ahani M., Rahimifard N., Shojaii A. Antibacterial activity of different extracts of aerial parts of Dionysia revoluta Boiss. against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella enteritidis, Enterococcus. Iranian Journal of Public Health. 2016;45:95.
- Mehrara M.,Rahimifard N., Hakemi-Vala M., Asgarpanah J.,Heidary M. A survey on the antimicrobial effect of methanol extract and alkaloid fractions of aerial parts of Glaucium vitellinum against different bacterial strains and Candda Spp with disk diffusion and MIC. Iranian Journal of Public Health. 2014;43:28.
- Hafezian M., Asgarpanah J.,Rahimifard N. Chemical Composition and Antimicrobial Activity of the Essential Oil from the Endemic Species Pycnocycla bashagardiana Mozaff. Lat. Am. J. Pharm. 2016;35(7):1634-9. 13.
- Rahimifard N., Shojaii A.,Mahbobi M., Hafezan G. H., Bagheri F. Evaluation of antibacterial activity and flavonoid content of two Capparis species from Iran. Journal of Medicinal Plants. 2015;14(55):89-94 14.
- Rahimifard N., Bagheri E., Asgarpanah G., Balajadeh B. K.,Yazdi H. R. 15 Antibacterial Activity of Total Extract, Petroleum Ether, Chloroform, Ethyl Acetate and Aqueous Fractions of Aerial Parts of Heliotropium bacciferum. 16. Journal of Medicinal Plants. 2014;13(52):122-135.
- Fournier P. E., Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006;42:692-9.
CrossRef - Kim N. J., Choi S. H et al. Risk factors for acquisition of imipenem-resistant Acinetobacter baumannii. a case-control study. Antimicrob Agents Chemother. 2004;48:224-8. 19.
- Antunes L. C., Imperi F., Carattoli A., Visca P. Deciphering the Multi factorial Nature of Acinetobacter baumannii Pathogenicity. PLoS One. 2011;6(8):226. 20.
- Rahimifard N.,Moghni M.,Naseri M. Evaluation and Comparison of Three Antimicrobial Activity Methods Using Bifidobacteria Bifidum and Bifidobacteria Infantis as Probiotic Bacteria against Salmonella enterica serotype Enteritidis. Journal of Bacteriology and Mycology: Open Access. 2016;2(3,00024):21.
- Shokraei S. S., Rahimifard N., Shad M. M. Comparison of three methods for evaluation of the antimicrobial activity of Lactobacillus acidophilus bacteria against Escherichia coli and Salmonella enterica serotype enteritidis. Iranian Journal of Public Health. 2014;43(167):22.
- Shokraei S. S.,Rahimifard N.,Shad M. M. Evaluation of the antimicrobial effect of Lactobacillus acidophilus isolated from probiotic infant formula on Escherichia coli. Iranian Journal of Public Health. 2014;43:152 23.
- Barzavar M., Rahimifard N. Evaluation of the Antimicrobial Activity of Lactobacillus gasseri as Probiotic Bacteria Against Salmonella enterica serotype Entertidis. GMP Review. 2015;16(4):56-64.

This work is licensed under a Creative Commons Attribution 4.0 International License.





