Manuscript accepted on : 25 March 2017
Published online on: --
Plagiarism Check: Yes
A Review of Genetic Taxonomy, Biomolecules Chemistry and Bioactivities of Citrus Hystrix DC.
Farid Agouillal1,2, Zarani M. Taher3, Houria Moghrani2, Noureddine Nasrallah2 and Hesham El Enshasy3,4,5
1Research Unit on Analysis and Technological Development in Environment (URADTE), Centre de Recherche Scientifique et Technique en Analyses Physico-Chimiques (CRAPC), Tipaza, Algeria.
2Laboratory of Reaction Engineering (LGR), Faculty of Mechanical Engineering and Process Engineering (FGMGP), Houari Boumediene University of Sciences and Technology (USTHB), Algiers, Algeria.
3Institute of Bioproduct Development (IBD), Universiti Teknologi Malaysia (UTM), Johor Bahru, Malaysia.
4Faculty of Chemical Engineering and Energy, Universiti Teknologi Malaysia (UTM), Johor Bahru, Malaysia.
5Genetic Engineering and Biotechnology Research Institute, City of Scientific Research and Technology Applications (CSAT), New Burg Al Arab Alexandria, Egypt.
DOI : http://dx.doi.org/10.13005/bbra/2446
ABSTRACT: Citrus hystrix DC. with common name makrut lime or kafir lemon, is a very popular traditional medicinal plant as well as an important spice in Asiatic countries. The plant is native of the Indonesian island Sumbawa, then, it is cultivated in Indonesia, Thailand, Malaysia and the tropical region of Asia. It mainly contains essential oil and phenolic compounds. The most intense odor compounds of kafir lemon are Citronellal, L-Linalool, 1,8-Cineole , α-Terpeneol and δ-Cadinene. Such as Citrusosides-A and furanocoumarines, Makrut lime content also non-volatile compounds like alkaloids and glyceroglycolipids. Citrus hystrix DC has many biological activities due to its volatile and nonvolatile compounds, and it has been used in traditional medicine for treating various illnesses, particularly cold pain and stomach disorder. It is also used as a juice for its fruit or as spice for its aromatic leaves. This review covers data on the chemistry and biological effects of Citrus hystrix DC biomolecules, and aims to lay the foundation for further study on the extraction enhancement of these biomolecules and more useful formulations.
KEYWORDS: Biomolecules chemistry; Biological activities; Citrus taxonomy; Citrus hystrix; Essential oils; Phenolic compounds
Download this article as:| Copy the following to cite this article: Agouillal F, Taher Z. M, Moghrani H, Nasrallah N, El-Enshasy H. A Review of Genetic Taxonomy, Biomolecules Chemistry and Bioactivities of Citrus Hystrix DC. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Agouillal F, Taher Z. M, Moghrani H, Nasrallah N, El-Enshasy H. A Review of Genetic Taxonomy, Biomolecules Chemistry and Bioactivities of Citrus Hystrix DC. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=22931 |
Introduction
Herbs and spices are extensively used in Asiatic countries for culinary purposes and for traditional medicine. Then, several organs such as the leaves and fruits of Citrus species are widely used to flavor foods as perfumes in ceremonial celebrations. Among these Citrus spices commonly planted in small garden plots of trees, C. hystrix D.C., C. aurantijolia Swingle, C. microcarpa Bunge, C. limn Burm. and C. mim Merr., are by far the most economically important plant. It is believed to have originated in south-eastern Asia, in an area that includes China, India and the Indochinese peninsula and nearby archipelagos.1
The genus Citrus belongs to the subfamily Aurantioideae and order of Sapindales of the Rutaceae family which comprises of about 140 genera and 1,300 species distributed between 7 subfamilies.2,3,4 The fruits and the leaves of the Citrus species contain a variety biologically-active compounds including essential oils with various distinct flavors which are important to human nutrition and diet, vitamin C, folic acid, potassium, flavonoids, coumarins, pectin, and dietary fibers.5
The name Mauritius papeda, or Kaffir lime, is widely used in the Netherlands and Germany while in France, Italy and Spain, the lime is often called ‘combava’. Later in 1824, the name “Mauritius papeda” was introduced to Kaffir lime by De Candolle (DC), who brought the seeds from Mauritius to his botanical garden in Montpellier in southern France. Before that time, De Candolle had studied and classified C. hystrix as the first species of the Papeda sub-genus.6
Genus Citrus is native to tropical south-east Asia, southern China and Malaysia. It has been introduced and cultivated elsewhere in the tropics and sub-tropics including in northern Australia.7,8 However, due to its aromatic, strong, unique and spicy flavor, both fruit and leaves of C. hystrix are popular used ingredient in Asian cooking. They are frequently used for instance in soups, curries, or to add flavour to rice. It can also be used as an infusion for both alcoholic and non-alcoholic drinks. In addition, the leaves can be used fresh or dried, and can be stored frozen.8 In Thailand, the fruit is used for seasoning and to prepare drinks teas such as Citrus hystrix flavonoid-rich sachet, which has been promoted to have great potential as a natural antioxidant health product. The essential oil is normally produced from fresh leaves by steam distillation and serves as a source of kaffir lime leaf flavours and essences in a large variety of internationally marketed products. The main producers of kaffir lime leaves are Thailand, Indonesia, Malaysia and India. Recently, Thai growers have developed and started growing a kaffir lime without wrinkles that is easier to pack and ship around the world.7,8
In aromatic plants, the composition of essential oils usually varies considerably because of intrinsic (sexual, seasonal, ontogenetic, and genetic variations) and extrinsic (ecological and environmental aspects) factors.9,10
Recent studies on the Malaysian Citrus plants have reported the identification and composition of essential oils of several Citrus species including C. aurantifolia, C. grandis, C. hystrix, and C. microcarpa5. Due to its high content of phenolic and flavonoid compounds, studies showed that citrus peel could be, also, potential source for natural antioxidant. In general, three types of flavonoid compounds are found on citrus peel; flavanone (e.g. hesperidin, naringin, and hesperitin), polymethoxylated flavone (e.g.nobiletin and tangeretin), and flavonol (e.g. rutin).11,12
In Europe, North America, Asia and Australia, dried kaffir lime leaves are available in most of the marts. Usually, a bag of dried leaves can be stored in a sealed airtight container for a couple of years with little physical change. However, the quality of the dried kaffir lime leaf product depends mostly on the drying methods that are industrially employed. The leaves can be harvested all year round, especially when the trees are small6. In addition to these uses, the hesperidium is still used to wash hair (as was noted in the seventeenth century by Rumphius) and, in Sri Lanka, to keep off leeches, apparently reflecting its insecticidal compounds having a rather broad range of effects on invertebrates.6,13 The leaves can be used to treat stomach ache caused by dyspepsia and insect bites; also, the rind has been prescribed for treatment for worms and headache.8, 14 In Peninsular Malaysia, the fruit and leaves have been also used for washing hair; the fruit is halved and the grated rind is rubbed on the head or the whole fruit is boiled and used as shampoo8; In addition, the fruit juice is used in softening the skin and the mixture of the fruit juice with bath water can be used to eliminate body odor.15 In traditional Medicine, C. hystrix is also used to treat flu, fever, hypertension, abdominal pains, and diarrhea in infants.16
The fruits are used as a digestive stimulant, blood purifier, and reduce high blood pressure.17,18 In Malaysia, the oils from the fruits and the leaves of C. hystrix DC are commercially used as flavors and fragrances, as well as in cooking, perfumery and medical treatments, especially in aromatherapy.19 This review aims to summarize the main studies reporting the chemical composition of essential oils and other extracts from the kaffir lime leaf , the used extraction processes, and discus their main biological activities. Finally, the formulation and safety level are considered.
Botany and Plant Taxonomy
Citrus hystrix is generally a small tree of 3–6 m high and a width of 2.5–3 m, often not straight, crooked with glabrous and spiny branches (Fig. 1). Leaves of kaffir lime are unique among the citrus varieties, they are alternate, unifoliolate, broadly ovate to ovate-oblong, 7.5–10 cm long, dark green on top, lighter on the bottom, very fragrant with long petiole expanded into prominent wings, 15 cm long by 5 cm wide, then, each leaf comes in two parts, seemingly a double leaf (Fig. 1.e). Leaf and expanded petiole appear to be a single “pinched” leaf. Leaf base is cuneate, or rounded, apex obtuse or slightly acuminate or notched.6,8 Flowers (Fig. 1.d) are small, fragrant, white; calyx cuspidate 4-lobed, white with violet fringe; petals 4–5, ovate-oblong, yellowish white tinged with pink; stamens 24–30 free. Fruit is large, verrucose, warty or bumpy, globose, ovoid to elliptic, green turning yellowish-green when ripe, approximately 5–7 cm diameter, rind thick, pulp yellowish, very acid and bitter with wrinkle on the surface of fruit (Fig. 1.b) . Seeds are numerous, ridged, ovoid-oblong, 1.5–1.8 by 1–1.2 cm, monoembryonic with white cotyledons (Fig. 1.c).6,8
 |
Figure 1: Tree (a), Fruit (b), Seeds (c), Flowers (d) and Leaves (e) of Citrus hystrix. Click here to View figure |
Kaffir lime grows well in a warm subtropical or tropical climate and prefers well-drained, neutral to slightly acid soil and direct sunlight with ample moisture during the growing season.4 Citrus taxonomy is still controversial due to the large degree of morphological diversity found in the group, sexual compatibility between the species and apomixes in many genotypes.6 Based on morphological and phenotypic data, the two major classification systems currently used are those of Swingle and Reece (1967)6 and Tanaka (1977).20 According to Tanaka system, it is hypothesized that citrus is originated in Asia about 30 million years ago from C. hystrix, C. latipes, C. macroptera and C. combara.21 Based on both bootstrap analysis of RAPD, SCAR data and cpDNA markers, Nicolosi et al. (2000)21 have separated eight clusters as C. hystrix belong to Micrantha cluster with C. micrantha, C. macroptera and C. latipes, all belonging to subgenus Papeda. These results place C. hystrix in a cluster genetically distinct from the ‘citron’ cluster as showed in Fig. 2.21,22
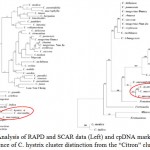 |
Figure 2: Analysis of RAPD and SCAR data (Left) and cpDNA markers (Right); Evidence of C. hystrix cluster distinction from the “Citron” cluster21. Click here to View figure |
Studying the genetic relationships among members of the Aurantioideae, especially of the genus Citrus and based on the chloroplast matK gene sequences analysis, Penjor et al. (2013)23 confirmed the results of Nicolosi et al. (2001)21 study that C. hystrix belongs to the Pummelo cluster which differs and stands out between the Mandarin cluster and the Citron Cluster as shown in Fig. 3.
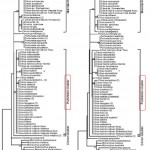 |
Figure 3: Chloroplast matK gene sequences analysis of genus Citrus; Maximum likelihood tree (Left) and neighbor-joining tree (Right) showing the three clusters: Citron, Pummelo and Mandarin. Penjor et al. (2013)23. Click here to View figure |
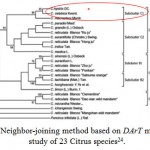 |
Figure 4: Neighbor-joining method based on DArT microarrays study of 23 Citrus species24.
|
However, Liu et al. (2015)24 reported the successful development of DArT microarrays and their applications in phylogenetic analysis of Citrus species, then, genetic relationships based on neighbor-joining method show that C. hystrix DC, C. macroptera Montr, and C. celebica Koord are grouped into subcluster C1 which is sister to another subcluster C2 including grapefruit (C. paradisi Macf.), pummelo (C. grandis (L.) Osbeck), lime (C. aurantifolia), indian wild orange (C. indica Tan.), citron (C. medica L.), and mandarin (C. reticulate) as shown in Fig. 4.
Chemical Composition
Based on chemical analysis, C. hystrix DC, is riche on bioactive molecules such as essential oils, phenolic compounds, glycerolipides and others; The main compounds of C. hystrix DC, will be described in the following paragraphs.
Essential oils
According to the International Standard Organization on Essential Oils (ISO 9235: 2013) and the European Pharmacopoeia, the term “essential oil” is reserved for a product obtained from vegetable raw material, either by distillation with water or steam, or from the epicarp of citrus fruits by a mechanical process, or by dry distillation.8 Essential oils are complex mixtures of volatile to semi-volatile compounds, usually with a strong odor, rarely colored, soluble in organic solvents, and insoluble in water. They comprise volatile compounds of terpenoid and non-terpenoid origin, synthetized through different biosynthetic routes and with distinct primary metabolic precursors. In nature, essential oils can be found in various plant organs (flowers, fruits, seeds, leaves, stems, and roots), and they play very important roles in plant defense and signaling processes.25,26 Also, essential oils are used as raw materials in many fields, such as pharmaceutical, agronomic, food, sanitary, cosmetic, and perfume industries.27
The essential oil of C. hystrix is used in aromatherapy and as essential ingredient of various cosmetic and beauty products; furthermore, the essential oil of C. hystrix has been reported to have various bioactivities such as antioxidant, antibacterial, antileukimic, and antitussive.28,29 Limonene, a monoterpene hydrocarbon, is the major component in the essential oils from the peels of the Malaysian Citrus species.30,31 In general, hydro diffusion steam distillation system, steam distillation with induction heating system, and automated steam distillation process with optimized temperature were applied to extract kaffir lime peel essential oil.32,33,34 From the fresh leaves of C. hystrix, the essential oil extracted by the steam distillation and the Likens-Nikerson extraction methods, was found to be dominated by citronellal (61.0%–73.0%), β-citronellol (10.0%–14.0%), and limonene (5.0%–7.0%) as major components.
In addition, citronellal (72.4%), β-citronellol (6.7%), and citronellyl acetate (4.1%) were reported to be the major components in kaffir lime leaves, followed by β-pinene (1.9%) and limonene (0.1%) as minor components5. Studying the chemical composition and antimicrobial activity of the essential oils from New Caledonian C. macroptera and C. hystrix from leaves, Waikedre et al. (2010),35 reported the presence of 38 constituents. The obtained essential oils were characterized by high contents of terpinen-4-ol (13.0%), β-pinene (10.9%), α-terpineol (7.6%), 1,8-cineole (6.4%), citronellol (6.0%) and p-cimene (5.6%), but poor in limonene (4.7%).
However, from the kaffir lime peels (from Masjid Tanah, Melaka in Malaysia), β-pinene (39.3%), limonene (14.2%), citronellal (11.7%), and terpinen-4-ol (8.9%) were identified as the principal components. Then, β-pinene (23.5%) and sabinene (20.1%) appeared as the major components followed by citronellal (12.6%), limonene (11.8%), and β-citronellol (3.3%) found in C. hystrix peel and reported by Nor (1999).36
Other study on essential oils extraction using automated steam distillation process with uncontrolled temperature carried out by Nurhani et al. (2013)34 reported that the oil composition was as follows: sabinene (31.224%), β-pinene (32.967%), limonene (20.687%), α-pinene (3.338%), camphene (0.135%) , myrcene (1.735%), α-terpineol (0.938%) and citronellal (7.531 %). Other compounds were identified using the same process but in the controlled temperature like terpinolene, linalool, terpinen-4-ol and citronellol. It was also reported that the essential oil isolated from Malaysian variety of kaffir lime peel contained sabinene (36.0% – 49.0%), limonene (17.0%–33.0%), citronellal (3.0%–11.0%), and β-pinene (8.0%–14.0%) as major components.37 However, citronellal (66.9%) and β-citronellol (6.6%) were the major components essential oil in kaffir lime peel (from Selangor), obtained using the hydro-distillation method. Other research also reported that the essential oil of fresh fruit-peel is mainly consisted of monoterpene hydrocarbons, with limonene (30.73%) and β-pinene (18.76%) as the principal components with other minor components such as terpinene-4-ol (10.63%), α-terpineol (8.35%), γ-terpinene (6.18%), α-terpinene (5.09%) and terpinolene (4.33%).38 In other study, citronellal was found to be the major component (80.04%) in C. hystrix leaf oil; In contrast, C. hystrix fruit peel essential oil consisted of other components: limonene (40.65%), terpinene-4-ol (13.71%) and α-terpineol (13.20%).39 Recent research of Aumeeruddy-Elalfi et al. (2016)40 found that the main compounds of essential oils from C. hystrix leaves as α-pinene (3.02%), limonene (83.89%), β-pinene (0.78%) and β-myrcene (0.89%) with traces of Methyl-eugenol (0.21%). From the study of Juraithip et al. (2010),41 we can draw another conclusion, that C. hystrix peel and leaf showed similar patterns of essential oils chemical compositions. The major constituents of C. hystrix peel and leaf were citronellal (about 23.85-23.41%) and trace components were elemol (6.59-4.17%), δ-cadinene (5.96-4.74), geranylacetate (5.12-4.45%), α-terpineol (5.15-5.40%), L-linalool (4.22-4.36%), β-pinene (1.82%), limonene (1.13%) and α-humulene (1.09-0.94%). The chemical structure of theses essential oils compounds are given in Fig. 5.
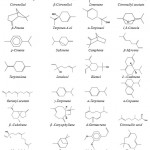 |
Figure 5: Structures of essential oils obtained from leaves and peels of C. hystrix
|
Table 1: Summary of main essential oils compounds from C. hystrix DC. leaves and peels as reported by previous studies.
| Location and years of study | Main components | % | Part of plant | references |
| Malaysia, 1996 | β-Pinene | 39.3 | Peels | 30 |
| Limonene | 14.2 | |||
| Citronellal | 72.4 | Leaves | ||
| Malaysia, 1999 | L-Citronellal | 61.73-72.45 | Leaves | 36 |
| 12.56 | Peels | |||
| L-Limonene | 5.90-6.78 | Leaves | ||
| 11.78 | Peels | |||
| Sabinene | 1.60-2.03 | Leaves | ||
| 20.13 | Peels | |||
| Linalool | 0.96-1.56 | Leaves | ||
| 1.82 | Peels | |||
| β-Citronellol | 10.34-13.43 | Leaves | ||
| 3.34 | Peels | |||
| Citronellyl acetate | 1.22-2.02 | Leaves | ||
| 1.67 | Peels | |||
| Thailand, 2007 | Limonene | 30.73 | Peels | 38 |
| β-Pinene | 18.76 | |||
| Terpinene-4-ol | 10.63 | |||
| α-Terpineol | 8.35 | |||
| γ-Terpinene | 6.18 | |||
| α-Terpinene | 5.09 | |||
| Terpinolene | 4.33 | |||
| New Caledonia, 2010 | Terpinen-4-ol | 13.0 | Leaves | 28 |
| β-Pinene | 10.9 | |||
| Limonene | 4.7 | |||
| α-Terpineol | 7.6 | |||
| 1,8-Cineole | 6.4 | |||
| Citronellol | 6.0 | |||
| Thailand, 2010 | Sabinene | 2.21 | Leaves | 41 |
| 1.55 | Peels | |||
| β-Pinene | 1.67 | Leaves | ||
| 1.82 | Peels | |||
| Limonene | 1.18 | Leaves | ||
| 1.13 | Peels | |||
| L-Linalool | 4.36 | Leaves | ||
| 4.22 | Peels | |||
| Citronellal | 23.41 | Leaves | ||
| 23.85 | Peels | |||
| α-Terpineol | 5.40 | Leaves | ||
| 5.15 | Peels | |||
| Citronellol | 1.40 | Leaves | ||
| 1.48 | Peels | |||
| Citronellyl acetate | 3.75 | Leaves | ||
| 3.82 | Peels | |||
| α-Copaene | 2.35 | Leaves | ||
| 2.16 | Peels | |||
| Geranyl acetate | 4.45 | Leaves | ||
| 5.12 | Peels | |||
| β-Cubebene | 2.46 | Leaves | ||
| 2.34 | Peels | |||
| β-Caryophyllene | 3.51 | Leaves | ||
| 3.73 | Peels | |||
| d-Germacrene | 1.82 | Leaves | ||
| 2.01 | Peels | |||
| δ-Cadinene | 4.74 | Leaves | ||
| 5.69 | Peels | |||
| Elemol | 4.17 | Leaves | ||
| 6.59 | Peels | |||
| Malaysi, 2011 | β-Citronellal | 66.85 | Leaves | 37 |
| β-Citronellol | 6.59 | |||
| Linalool | 3.90 | |||
| Citronellol | 1.76 | |||
| Thailand, 2012 | Limonene | 40.65 | Leaves | 39 |
| Terpinene-4-ol | 13.71 | |||
| α-Terpineol | 13.20 | |||
| Thailand, 2013 | Citronellic acid | 4.5 | Leaves | 29 |
| Nerolidol | 2.14 | |||
| δ-Cadinene | 1.49 | |||
| Citronellal | 1.41 | |||
| Citronellol | 1.39 | |||
| Malaysia, 2013 | Sabinene | 27.498 – 45.594 | Peels | 32 |
| Limonene | 28.649 – 32.455 | |||
| Citronellal | 8.293 – 17.487 | |||
| β-Pinene | 7.147 – 8.974 | |||
| α-Pinene | 1.255 – 2.515 | |||
| Malaysia, 2013 | Sabinene, | 46.165 – 48.5 | Peels | 23 |
| Limonene, | 25.742 – 27.714 | |||
| β-Pinene | 8.759 – 10.088 | |||
| Citronellal | 3.247 – 7.146 | |||
| α-Pinene, | 2.959 – 3.223 | |||
| Myrcene | 1.338 – 1.469 | |||
| Terpinen-4-ol | 0.457 – 1.054 | |||
| Malaysia, 2013 | Sabinene | 31.224 – 46.573 | Peels | 34 |
| β-Pinene | 13.509 – 32.967 | |||
| Limonene | 17.232 – 20.687 | |||
| Citronellal | 4.616 – 7.829 | |||
| α-Pinene | 3.047 – 3.546 | |||
| Myrcene | 1.804 – 1.985 | |||
| Terpinen-4-ol | 1.823 – 2.822 | |||
| γ-Terpinene | 0.735 – 1.775 | |||
| Linalool | 0.633 – 1.156 | |||
| α-Terpineol | 0.438 – 0.907 | |||
| Mauritius, 2016 | Limonene | 83.89 | Leaves | 40 |
| α-Pinene | 3.02 | |||
| β-Myrcene | 0.89 | |||
| β-Pinene | 0.78 |
Phenolic and Flavonoid Compounds
hystrix also function to scavenge radical activities, due to their phenolic compounds which has beneficial implications in human health, phenolic compounds (PC) are widely distributed in fruits and vegetables.42 In terms of chemical structure, phenolic compounds have at least one aromatic ring to which one or more hydroxyl groups are bonded to aromatic or aliphatic structures.43
Phenolic compounds range from simple phenolic molecules to highly polymerized compounds. However, these phenolic compounds were obtained mainly in ethanolic extracts.44
Besides their antioxidant activities, flavonoids have been demonstrated to have a wide range of biochemical and pharmacological effects including anti-inflammatory, anti-viral, anti-allergenic, anti-carcinogenic, anti-ageing activity, anti-oxidant and anti-allergic effects.45,46 Flavonoids represent the widely distributed group of plant phenolics, including the anthocyanin pigments, flavonols, flavones, flavanols, and isoflavones. The flavanols tend to polymerize to condensed tannins.47
The group of non-flavonoids is mainly represented by benzoic and cinnamic acid known as phenolic acids.44 Flavonoids are the most common and widely distributed group of plant phenolic compounds that are characterized by a benzopyrene structure, which is ubiquitous in fruits and vegetables; and can be analyzed using colorimetric method by reaction with sodium nitrite and the development of coloured flavonoid– aluminium complex formation using aluminium chloride. The presence of polyphenolic compounds like gallic acid, hesperidin, and naringin in citrus fruits have been suggested to be responsible for the anti-diabetic activity.48,49 Interestingly, the peels of C. hystrix have been reported to contain a variety of phenolic compounds, mainly flavanone, flavone and flavonol50. In C. hystrix, hesperidin is reported as component, which is responsible for radical scavenging activity.51,52
Using supercritical carbon dioxide extraction, vanillic acid, p-coumaric acid, sinapic acid, m-coumaric acid, benzoic acid and cinnamic acid were isolated from the plant leaves53. Three known coumarins, bergamottin, oxypeucedanin and 5-[(6’,7’-dihydroxy-3’, 7’-dimethyl-2-octenyl)oxy] psoralen were exhibited inhibitory activities against both lipopolysaccharide (LPS) and interferon- γ (IFN-γ )-induced nitric oxide (NO) generation in RAW 264.7 cells54. The chemical structures of these main phenolic compounds found in C. hystrix are showed in Fig. 6.55
Other Extracts
Two glyceroglycolipids were isolated by Murakami et al. (1995),56 from the leaves of C. hystrix, and identified as l,2-di-O-a-linolenoyl-3-O-beta-galactopyranosyl-sn-glycerol (DLGG) and a mixture of two compounds, l-O-a-linolenoyl-2-O-palmitoyl-3-O-beta-galactopyranosyl-sn-glycerol and its counterpart (LPGG). These compounds inhibit the tumor-promoting activity of 12-O-Tetradecanoylphorbol 13-Acetate in Mouse skin. However, in addition of two coumarins (hystrixarin and hopeyhopin, an benzenoid derivatives (hystroxene-I), and an quinolinone alkaloid (hystrolinone), as shown in Fig. 6, were isolated from the crude acetone extract of root of C. hystrix.57
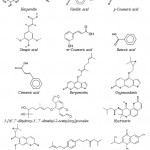 |
Figure 6: Structure of phenolic compounds, coumarins and a quinolinone alkaloid from C. hystrix DC.
|
Biological Activities
According to the diversity of chemical compounds extract from C. hystrix, several works have undertaken for the assessment of some biological activities both in vitro or in vivo systems.
Antimicrobial: Antibacterial and Antifungal Activities
Waikedre et al. (2010),35 have tested the leaves essential oil against three Gram positive bacteria (Staphylococcus aureus, Staphylococcus epidermidis and Bacillus subtilis), two Gram negative bacteria (Klebsiella pneumonia and Escherichia coli), and five fungal strains (Aspergillus fumigates, Candida albicans, Cryptococcus neoformans, Saccharomyces cerevisiae and Trichophyton mentagrophytes). The tested essential oil was inactive against bacteria but showed moderate activity against Cryptococcus neoformans and Saccharomyces cerevisiae with MIC of (50 mg/ml). This value is about tenfold lower than the used antifungal agent standard (ketoconazole :5 mg/ml). The GC-MS of the tested essential oil was characterized by high contents of terpinen-4-ol (13.0%), a-terpineol (7.6%), 1,8-cineole (6.4%), and citronellol (6.0%). Testing the antibacterial activities of the two essential oils of makrut leaf and makrut fruit peel against 411 isolates of groups A, B, C, F, G Streptococci, Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus (Methicillin-Resistant and -Sensitive S. aureus) and Acinetobacter baumannii, obtained from patients with respiratory tract infections, Vimol et al. (2012),39 report that both essential oils were effective against all tested pathogens with minimal inhibitory concentration (MIC) ranges of 0.06–68 mg/ml and 0.03–17.40 mg/ml, respectively for leaves and fruit essential oils from C. hystrix. The GC-MS analysis of the used essential oils revealed that citronellal was found to be the major component (80.04%) in the leaf essential oil and had the lowest MIC. In contrast, fruits peel essential oil consisted of mixture of components (limonene 40.65%, terpinene-4-ol 13.71%, α-terpineol (13.20%), and the most active fraction was α-terpineol, followed by terpinene-4-ol, and limonene.39 The antimicrobial activities of volatile oils and extracts of eight Thailand species, C. hystrix essential oil were investigated against eight bacteria (3 Gram positive bacteria, Bacillus subtilis (ATCC 6051), Staphylococcus epidermidis (ATCC 12228), S. aureus (ATCC25923); 5 Gram negative bacteria: Escherichia coli (ATCC 25922), Enterococcus faecalis (ATCC 1406), Proteus mirabilis (ATCC 14153), Pseudomonas aeruginosa (ATCC 27853); Mycobacterium: Mycobacterium phlei (ATCC 11758) ) and three fungi (Candida albicans (ATCC 10231), C. parasilosis (ATCC 90018) and C. tropicalis (ATCC 13803).41
The volatile oil of C. hystrix leaf, did not show any inhibitory activity against tested organisms, but interestingly, growth of Mycobacterium phlei was inhibited by the volatiles of C. hystrix peel with MIC of 3.5 mg/ml, with the similar patterns of essential oils in both C. hystrix peels and leaves. This bioactive fraction composed of citronellal (about 23%) and similar trace components as L-linalool (4.22%), β-pinene (1.82%) and limonene (1.13%).41
It have been also reported hydrodistillation and ethyl acetate extract of C. hystrix peels showed broad spectrum of inhibition against three Gram-positive bacteria (Staphylococcus aureus, Bacillus cereus and Listeria monocytogenes), one yeast (Saccharomyces cerevisiae var. sake) and one mold (Aspergillus fumigatus TISTR 3180).58 The tested ethyl acetate extracted essential oils of C. hystrix peel had stronger antibacterial activity than the volatile obtained from hydrodistillation.
It exhibited minimum inhibitory concentration (MIC) values of 0.28 and 0.56 mg/ml against the tested yeast and B. cereus, respectively while the minimum bactericidal concentration (MBC) values against both microbes were 0.56 mg/ml. The MIC values of the ethyl acetate extracted essential oils against L. monocytogenes, S. aureus and the mold were 1.13 mg/ml while the MBC values against L. monocytogenes as well as the mold A. fumigatus TISTR 3180 and S. aureus were 2.25 and 1.13 mg/ml, respectively. The GC-MS analyses revealed that the major components of the ethyl acetate extracted essential oil were limonene (31.64 %), citronellal (25.96 %) and β-pinene (6.83 %) whereas β-pinene (30.48 %), sabinene (22.75 %) and citronellal (15.66 %) appeared to be major compounds of the essential oil obtained by hydrodistillation.58
Anti-Inflamatory and Antioxidant Activities
On studying anti-Inflammatory response with a model based on lipopolysaccharide-activated RAW 264.7 Murine Macrophages, Tuntipopipat et al. (2009),59 found that among 13 plants, the extract (with 70% ethanol ) of the freeze-dried fresh leaf C. hystrix, with extract from seven other plant, inhibited NO and TNF-a production in a dose-dependent manner without exerting cytotoxicity. Kaffer lime extract should be used in a concentration of 29.2+2.1 µg/mL and 35.4+1.5 µg/mL to reach the IC50, respectively for the inhibition of NO Production and for TNF-a Secretion by LPS-Activated RAW 264.7 Cells.
For antioxidant activity evaluation, based on DPPH radical scavenging capacity method, the methanolic extracts from leaf and peel of C. hystrix, have promising a potent antioxidant activity with IC50 of 24.6 and 66.3 microg/ml respectively for leaves and peel.41 The antioxidant activity of fresh juice of C. hystrix was evaluated by employing different in vitro assays covering applied for the contents of total phenolics, tannins, and total flavonoids ranged that tanged respectively 836.90 mg gallic acid equivalent (GAE)/L, 507.61 mg gallic acid equivalent(GAE)/L and 224.88 mg rutin equivalent/L. Antioxidant potential based on FRAP assay show a value of 30504.40 mmol of ferrous equivalents/L juice, DPPH• and ABTS•+ scavaging are respectively about 10903,28 mmol of trolox equivalents/L juice, and 33830.69 mg of EDTA equivalents/L juice. Both superoxide radical and hydroxyl radical scavenging activity are in the range of 19.89 % and 42.91 %, respectively. Also, metal chelating activity reaches 7.73 mg of EDTA equivalents/L juice. These results indicated that fresh juice of C. hystrix could be used as a source of antioxidant agents.60
Hepatoprotective Activity
Abirami et al. (2015)61 have evaluated the hepatoprotective effects of C. hystrix methanolic leaf extracts on paracetamol induced toxicity in a Swiss albino mice model. Leaf extracts were administrated at the dose of 200 mg/kg body weight for 7 days and toxicity was induced by paracetamol (2 g/kg) on day 5, Liver function markers (ALT, AST, ALP), total bilirubin and total protein in blood serums and hepatic antioxidants (SOD, CAT, GSH and GPx) in liver homogenate were estimated after that animals were sacrificed on the 7th. day.
However, the recent study conducted by Abirami et al. (2015),61 shows that methanolic extracts of C. hystrix leaf possess hepatoprotective action against murine paracetamol induced hepatotoxicity; The level of enzyme markers (alanine transaminase, aspartate transaminase and alkaline phosphatase) in experimental rats were significantely restorated of by the interventions C. hystrix leaves extract to the comparable level of normal control.
Pretreatment with C. hystrix extracts brought back the oxidative stress markers (superoxide dismutase, catalase and glutathione peroxidase) in the range of normal control rats. In the same study, the histopathological examination have confirmed that pretreatment with methanolic extracts of C. hystrix leaf in paracetamol intoxicated rats showed recovery of the hepatocytes from necrosis indicating that sample extracts preserved the structural integrity of the hepatocellular membrane and liver cell architecture damaged by paracetamol action.
Anti-Cancer Effect
The early study to assess the anticancer properties of methanolic extract of C. hytrix fresh leaves by Murakami et al. (1995)55 reported the plant anti-tumor properties on mouse skin in a two-stage induced by dimethylbenz[a]anthracene (DMBA) and 12-O-tetradecanoylphorbol 13-acetate (TPA). The results showed that the IC50 values of two compounds were strikingly lower than those of representative used cancer preventive agents such as a-linolenic acid, beta-carotene, or (-) epigallocatechin gallate. Also, one compound exhibited anti-tumor-promoting activity was identified as l,2-di-O-a-linolenoyl-3-O-beta-galactopyranosyl-sn-glycerol (DLGG). The second compound was identified as a mixture of l-O-a-linolenoyl-2-O-palmitoyl-3-O-beta-galactopyranosyl-sn-glycerol and its counterpart (LPGG) as shown in Fig 7.
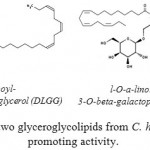 |
Figure 7: Structure of two glyceroglycolipids from C. hystrix with anti-tumor promoting activity.
|
However, its well known that several medicinal plant exhibit anti-proliferative activity,62 Manosroi et al.(2006),63 have investigated the anti-proliferative activity of essential oil extracted from 17 Thai medicinal plants on human mouth epidermal carcinoma (KB) and murine leukemia (P388) cell lines using MTT assay. The IC50 value of both C. hytrix fruit and leaf, was, respectively, 0.0997 – 1.1479 mg/ml in KB cell line and 0.0746- 0.3977 in P388 cell line. Then, these two C. hytrix essential oils have an anti-proliferative activity on cervical cancer (KB cell) and mouse leukemia (P388 cell).
Five fractions of crude extract (hexane, ethanol, ethyl acetate, butanol and methanol) from the leaves of C. hystrix were investigated in vitro for their potential cytotoxic activity on 4 leukemic cell lines (HL60, K562, Molt4, U937), and normal human peripheral blood mononuclear cells (PBMCs) using the MTT assay.64
The cytotoxicity bioassays showed that the ethyl acetate fraction exhibited the highest cytotoxicity, with IC50 values of 19.0±0.6, 35.3±1.4, 21.8±0.4, and 19.8±1.0 μg/ml, in response to the 4 leukemic cell lines (HL60, K562, Molt4, and U937), respectively.
These were higher than those of fractions from hexane, ethanol, and butanol. However, none of the five fractions had cytotoxic effects on PBMCs; Also, the methanol fraction did not exhibit any cytotoxic activity.64
The cytotoxicity effects and apoptosis induced by three different kaffir lime leaves extract (ethanol, ethyl acetate, and hexane) to cervical cancer cell line (HeLa cells) were studied by Nastiti et al. (2013).65 They used cytotoxicity assay via MTT assay, and apoptosis test with double staining method (ethidium bromide-acrydine orange). The three kaffir lime crude extract exhibited dose dependently HeLa cells proliferation inhibition. The IC50 of ethanolic and ethyl acetate extract was 82,034 and 57,845 μg/mL, respectively, these two extract were able to induce apoptosis of HeLa cells by increasing the number of apoptotic cells. On the other hand hexane extract was not cytotoxic with lC50 of 203, 992 μg/mL. In addition, the results showed that ethyl acetate extract of kaffir lime was the most potential to induce apoptosis in HeLa cells.
The cytotoxic effect of kaffir lime leaf extracts on cervical cancer and neuroblastoma cell lines based on the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was carried out by Woro et al. (2014)66. They showed that both ethyl acetate and chloroform extracts have an IC50 for HeLa cells, UKF-NB3, IMR-5 and SK-N-AS parental cells of 40.7-17.6; 28.4-18.9; 14.1-6.4 and 25.2-9.4 (μg/mL) respectively. Then, kaffir lime extract reduces the viability of cervical and neuroblastoma cell lines and may have potential as anti-cancer compounds.
Cholinesterase Inhibition Activity
Increasing communication between nerve cells that use acetyl-choline as a chemical messenger produce a therapeutic effect in patients with Alzheimer’s disease, glaucoma, myasthenia gravis, and for the recovery of neuromuscular block in surgery, then, acetylcholine breakdown in the brain can be prevented by the inhibition of acetyl cholinesterase activity, which subsequently increases the concentration of acetylcholine.67 The juices of C. hystrix possess strong anti-cholinesterase activity of 79.74% against 86.89% of the used reference compound (Eserine).60
From the hexanes and dichloromethane extracts of the peels of C. hystrix fruits, Youkwan et al. (2010)68 have isolated 4 new citrusosides A-D, six furanocoumarins, a sesquiterpene (eudesmane-4b,11-diol), 5 monoterpenes, and 1-O-isopropyl-beta-D-glucopyranoside. The Butyryl-cholinesterase inhibitory activity of the isolated fractions was investigated and 6′-hydroxy-7′-methoxybergamottin was found as a compound to possess the highest potency, showing an IC50 value of 11.2+/-0.1 μM against 3.2+0.2 μM of galanthamine (a positive control). However, 6′,7′-dihydroxybergamottin and isoimperatorin showed IC50 values of 15.4+/-0.3 and 23+/-0.2 μM, respectively. These molecules are represented in Fig. 8.
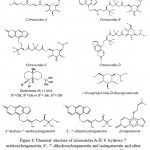 |
Figure 8: Chemical structure of citrusosides A-D; 6’-hydroxy-7’-methoxybergamottin; 6’, 7’-dihydroxybergamottin and isoimperatorin and other molecules responsible of cholinesterase inhibition activity58.
|
In the study undertaken by Wantida et al. (2010),69 essential oils of C. hystrix were tested for their acetyl-cholinesterase (AChE) and butyryl-cholinesterase (BChE) inhibitory activities. The tested essential oil exhibited inhibitory activity on BChE higher than on AChE.
Among sixteen compounds isolated by Chonticha et al.(2016),70 from the ethyl acetate extract of the fruit peels of C. hystrix, only one flavonol compound, (3-O-beta-D-glucopyranosyl-3,5,7,4’-tetrahydroxy-6,8,3’-trimethoxyflavonol nucleus in the prenylfuranocoumarin–HMGA conjugate, Fig. 9) showed very potent butyryl-cholinesterase inhibitory activity with IC50 value of 10.12 ± 0.22 µM, against galanthamine, a positive control compound with IC50 value of 11.2 ± 0.09 µM.
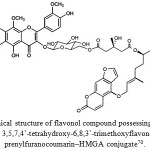 |
Figure 9: Chemical structure of flavonol compound possessing a 3-O-beta-D-glucopyranosyl- 3,5,7,4’-tetrahydroxy-6,8,3’-trimethoxyflavonol nucleus in the prenylfuranocoumarin–HMGA conjugate70.
|
Insecticidal and larvicidal Activities
The study of insecticidal properties of essential oil from Citrus hystrix DC fresh leaves against tobacco armyworm Spodoptera litura fabricius, using topical application bioassay on uniform weighted second instar larvae, demonstrated considerable repellant activity against the armyworm larvae after 24 and 48 h of treatment with LD50 values of 29.25 and 26.75 µg/mL, respectively. Also, the growth and development study in the antifeedant test showed that weight gained of larvae treated with C. hystrix essential oil were lower as compared to control treatment.
GCMS analyses of the tested essential oil revealed the presence of 29 compounds with dominance of beta-citronellal as major compound (66.85%) of total essential oil followed by beta-citronellol (6.59%), linalool (3.90%) and citronellol (1.76%).37
In another study, Mya et al. (2015),71 used ethanol extract of Citrus hystrix leaves to assess their larvicidal effects against Aedes aegypti which is the primary vector of dengue72; Result suggests that high concentrations of Citrus hystrix leaves ethanol extract can be used for the eradication of A. aegypti; then, concentrations of 2.4-2.1-1.8-1.5 and 1.2% of the tested leaves ethanol extract caused 99.5-85.5-62.5-26.5 and 2% mortality of Aedes larvae in 24 hrs, respectively.
Ansori et al. (2015),73 tested (methanol) and non-polar (n-hexane) extract fractions of C. hystrix leaves, with concentrations of 500 ppm, 1375 ppm, 2250 ppm, 3125 ppm, and 4000 ppm against the 3rd instar larvae of A. aegypti; The number of mosquito larvae mortality was calculated after 24 hours of treatment. The results reported that non-polar extract fraction is more toxic and is an effective biolarvicide with LC90 = 2,885 ppm compared with polar extract fraction which has an LC90 = 3,180 ppm.
Using an excito-repellency test system, Nararak et al. (2016),74 studied the effect of essential oils of the leaf and peel of kaffir lime at four different concentrations (0.5, 1.0, 2.5, and 5.0% v/v) for their repellency, excitation, and knockdown properties against laboratory strains of A. aegypti (L.) and Anopheles minimus Theobald at the 3–5 day aged old mosquito starved 24 h before testing.
For repellency against A. aegypti, leaf volatile oil produced the greatest response for both contact (56.1% escape) and non-contact trials with 63.3% escape at 2.5%, while peel volatile oil produced the strongest response with 46.5% escape at 2.5%.
Against Anopheles minimus Theobald, essential oil from C. hystrix leaf had strong combined irritant and repellent activity responses at 1–5% concentrations (90.0–96.4% escape) and the strongest spatial repellent activity at 1% and 2% (85.9% and 87.2% escape), respectively. The peel essential oil exhibited good excitation with repellency at concentrations of 2.5% (89.8% escape) and 5% (96.28% escape), while concentrations 1–5% showed more moderate repellent activity.
However, knockdown responses above 50% were only observed in A. aegypti exposed to 5% leaf essential oil. Then, the tested Kaffir lime essential oils were more active against Anopheles minimus Theobald than A. aegypti mosquitoes.74 Leaf significantly appear more active then peel essential oils at each concentration against Anopheles minimus in contact and non-contact trials, except at the highest (5%) concentration.
Others Bioactivities: Antifertility, Tyrosinase Inhibitory Activity and Cardioprotective Effect
Pawinee et al.(1995)75 investigated the effect of oral adminstration of both alcohol and chloroform extract of C. hystrix DC fruit peel for antifertility activity in pregnant adult female rats (Wistar) by oral administration at different periods of gestation.
They showed an enhancement of the uterotrophic effect of estradiol when both extract were simultaneously given; additionally, the extract stimulated uterine contractions. These two effects may be responsible for the interruption of pregnancy associated with the extract. Then, alcohol and chloroform extract of C. hystrix were found to effectively inhibit implantation, produce abortion and slightly hasten labor time when it was given from day 2 to 5, day 8 to 12 and day 15 until labor, respectively.
Administration of the chloroform extract in a dose of 1 g/kg produces a 62.2 f 14.5% inhibition of implantation. However, administration of the chloroform extract at a dose of 1 g/kg twice a day from day 8 to 12 interupted pregnancy by 91.9+5.5% while the same amount of the alcohol extract produced the effect by 86.3+ 9.6%.
According to the anti-implantation effect, they founded that chloroform extract also possesses a higher abortifacient activity than the alcohol extract.75
Tyrosinase is responsible for the formation of melanin in the human body; however, surplus expression of tyrosinase is a major problem which can lead to several skin hyperpigmentation disorders such as, seborrheic keratoses, melasma, diabetic dermopathy, tinea versicolor, melasmas and malignant melanomas. 76Abirami et al. (2014),60 reported that C. hystrix juice exhibited excellent tyrosinase inhibitory activity of 80.79%, against 90.87% of the used reference compound (Kojic acid).
A recent study conducted by Aumeeruddy-Elalfi et al. (2016)77 showed the potency of 19 essential oils from exotic and endemic medicinal plants from Mauritius. The results tend to show that essential oils extracted from these medicinal plants can exhibit anti-tyrosinase activities and may be potential candidates for the cosmetic, food and pharmaceutical industries. Results showed that C. hystrix essential oils exhibit an IC50 of 2.08 ± 0.253μg/ml. Putri et al. (2013),78 analyzed the effects of C. hystrix peel ethanolic extract on blood serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity, and observed by light microscope the cardio-hepato-histopathology of a doxorubicin-induced cardiac and hepatic toxicity animal model (female Sprague Dawley rats). In the animal groups receiving 500 mg/kg to 1000 mg/kg C. hystrix peel ethanolic extract, cardiohistopathology profile of doxorubicin induced cardiotoxicity and hepatotoxicity rats was repaired, but neither hepatohistopathology profile was did repaired nor serum activity of aminotransferase (ALT) and aspartate aminotransferase (AST) was reduced; Thus, Putri et al. (2013),78 conclude that the ethanolic extract of C. hystrix, can be developed as cardioprotector agent.
Safety issues
Regarding the chemical structures and the biological activities of biomolecules from C. hystrix, and also, the several domestic uses of this plant and their extracts, it appears that a bio-safety issue for this plant may be highlighted.
No information was founded on toxicity profile of C. hystrix biomolecules in the second edition of a Guide for Health Care Professionals of Essential Oil Safety.79
From, the released draft tentative report of the Cosmetic Ingredient Review Expert Panel (May and October, 2016), entitled ‘‘Safety Assessment of Citrus Flower- and Leaf-Derived Ingredients as Used in Cosmetics’’. Thus, it appear that C. hystrix leaf extract produced by extracting dried leaves with 80% ethanolic solution is reported as safe and to be non-irritating and non-sensitizing.
Conclusion
hystrix has been used as food and medicine with long history mainly in the Asian region. It is also a flavor food with health value. The present study reported phylogenetic taxonomy based on bootstrap analysis of RAPD, SCAR data, cpDNA markers and the chloroplast matK gene sequences analysis showed that C. hystrix belong to Pummelo cluster which is genetically distinct from the Citron cluster and the Mandarin cluster. The chemical structures of bioactives molecules explain its traditional uses and his potential to be used in cosmeticeucal and pharmaceuticals. hystrix essential oils are the mostly studied biomoleculs and they have potential beneficial therapeutic actions in the management of bacterial and fungal infections. The chemical composition revealed that essential oil of leaves and fruit peel of C.hystrix have generally a different profile; then, amoung reported studies, the C.hystrix leaves are characterized by citronellal, β-citronellol and terpinen-4-ol as major components. However, citronellyl acetate, β-pinene, limonene, alpha-terpineol, 1,8-cineole, citronellol, p-cimene, and limonene were identified as minor components. Whereas, kaffir lime peels content respectively limonene, β-pinene, sabinene and citronellal as major components with other minor components like terpinene-4-ol, a-terpineol, g-terpinene, a-terpinene and terpinolene. Other essential oils compounds of C. hystrix were detected in little amount in both leaves and peels such as elemol, delta-cadinene, geranylacetate and L-linalool. The reported studies show that some of tested essential oils were inactive against bacteria. Mainly those content terpinen-4-ol as major compounds. However, essential oils with citronellal as major component were more effective against bacterial strains.
In the future, more deep analysis and profiling of the volatile oils are needed to allow further elaboration of a chemotype of C. hystrix based on essential oils profile. Interestingly, phenolic compounds of the peels of C. hystrix contain a variety of flavanone, flavone and flavonol; Vanillic acid, p -coumaric acid, sinapic acid, m –coumaric acid, benzoic acid and cinnamic acid were isolated from C. hystrix leaves. In addition, flavonoids such as cyanidin, myricetin, peonidin, quercetin, luteolin, hesperetin, apigenin and isorhamnetin, as flavanone compounds, didymin and hespiridine were isolated from both leaves and fruit juice and as flavone compounds, rutin and diosmin were isolated just from the leaves. Glyceroglycolipids, l,2-di-O-a-linolenoyl-3-O-beta-galactopyranosyl-sn-glycerol (DLGG) and a mixture of l-O-a-linolenoyl-2-O-palmitoyl-3-O-beta-galactopyranosyl-sn-glycerol and its counterpart (LPGG) were identified in the C. hystrix leaves. Benzenoid derivatives, (hystroxene-I), quinolinone alkaloid (hystrolinone) were isolated from the crude acetone extract of root of Citrus hystrix.
With diversity of contents in the total phenolics, both ethanolic and methanolic extract have promising a potent antiinflamatory, antioxidant activity and hepatoprotective effects. Fresh leaves methanolic extract content l,2-di-O-a-linolenoyl-3-O-beta-galactopyranosyl-sn-glycerol (DLGG) which appear an anti-tumor-promoting agent. Also, some essential oils extracted from fruit and leaves C.hytrix have an anti-proliferative activity on cervical cancer (KB cell) and mouse leukemia (P388 cell). Also, Ethyl acetate fraction of leaves of kaffir lime exhibited the highest cytotoxicity activity on leukemic cell lines inducing, also, highest apoptosis in HeLa cells and reducing the viability of cervical and neuroblastoma cell lines. Cholineserase inhibitory activity was manifested by juices, hexane and dichloromethane extracts of C. hystrix, the implicated molecules are citrusosides compounds and 6’-hydroxy-7’-methoxybergamottin and a compound with 3-O-beta-D-glucopyranosyl- 3,5,7,4’-tetrahydroxy-6,8,3’-trimethoxyflavonol nucleus in the prenylfuranocoumarin–HMGA conjugate.
Essential oil from C. hystrix DC fresh leaves showed larvicidal effects against tobacco armyworm Spodoptera litura fabricius; and a repellant effect, with the peel essential oils, against adult mosquito of Aedes aegypti (L.) and Anopheles minimus Theobald, However, larvicidal effects against Aedes aegypti is reported with the ethanolic extract of Citrus hystrix leaves. Others bioactivities such as antifertility effect, tyrosinase inhibitory activity, cardioprotective and hepatoprotective effects were reported. Alcohol and chloroform extract of C. hystrix were found to effectively inhibit implantation and produce abortion, therefore, the antifertility effect were proposed. Both essential oils and juice of C. hystrix exhibited excellent tyrosinase inhibitory activity and the peel ethanolic extract, showed cardiotoxic and hepatotoxic protective effects on rat model. Both emulsions and microcapsules have been described for the formulation of C. hystrix essential oil. Finally, we think that the safety issues in terms of toxicity profile related to the daily use of these biomolecules should be further investigated. In addition, further studies on non-conventional extraction processes involving less solvent and energy use are also needed.
References
- Krueger R. R., Navarro L. Citrus germplasm resources. In: Khan IA. ed. Citrus genetics, breeding and biotechnology. Wallingford, UK: CAB International. 2007;45–140.
CrossRef - Hynniewta M., Malik S. K., Rao S. R. Genetic diversity and phylogenetic analysis of Citrus (L) from north-east India as revealed by meiosis, and molecular analysis of internal transcribed spacer region of rDNA. Meta Gene. 2014;2:237-251.
CrossRef - Kamal G. M., Anwar F., Hussain A. I., Sarri N., Ashraf M. Y. Yield and chemical composition of Citrus essential oils as affected by drying pretreatment of peels. Food Res. J. 2011;18(4):1275-1282.
- Bown D. The Royal Horticultural Society New Encyclopedia of Herbs & Their Uses, Great Britain, Dorling Kindersley, London. 2002;448.
- Md Othman S. N. A., Hassan M. A., Nahar L., Basar N., Jamil S., Sarker S. D. Essential oils from the Malaysian Citrus (Rutaceae) Medicinal Plants. Medicines. 2016;3(2):13. doi:3390/medicines3020013
- Swingle W. T., Reece P. C. W., Webber H. J and Batchelor L D. (eds). The botany of citrus and its wild relatives, in Reuther The Citrus Industry, University of California Press, Berkeley, CA. 1967;1:190–430.
- Manner H. I., Buker R. S., Smith V. E., Ward D., Elevitch C. R. Citrus (citrus) and Fortunella (kumquat) species profile. Isl. Agrofor. 2006;2:1–35.
- Lim T. K. Edible Medicinal and Non-Medicinal Plants.Fruits. 2012;4:1023.
- Taiz L., Zeiger E. Plant physiology, 5th Sinauer Associates Inc. Publishers. 2010;778.
- Figueiredo A. C., Barroso J. G., Pedro L. G., Scheffer J. J. C. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Fragr. J. 2008;23:213–226.
CrossRef - Choi S. Y., Ko H. C., Ko S. Y., Hwang J. H., Park J. G., Kang S. H., Han S. H., Yun S. H., Kim S. J. Correlation between flavonoid content and the NO production inhibitory activity of peel extracts from various Citrus Biol. Pharm. Bull. 2007;30:772-778.
CrossRef - Peterson J. J., Beecher G. R., Bhagwat S. A., Dwyer J. T., Gebhardt S. E., Haytowitz D. B., Holden J. M. Flavanones in Grapefruit, Lemons, and Limes: A compilation and review of the data from the analytical literature. Food Compos. Anal. 2006;19:74-80.
CrossRef - Koh D., Ong C. N. Phytophotodermatitis due to the application of Citrus hystrix as a folk remedy. J. Dermatol. 1999;140:737–738.
CrossRef - Wongpornchai S. Kaffir lime leaf. In: Handbook of Herbs and Spices (Second edition K. V. Peter), Wood head Publishing Series in Food Science. Technology and Nutrition.2012;319-328.
CrossRef - Dassanayake M. D in: A Revised Handbook to the Flora of Ceylon; Amerind Publishing Co Ltd. New Delhi, India., 1985;5:432–433.
- Fortin H., Vigora C., Lohezic-Le F., Robina V., Bosse B. L., Boustiea J., Arnoros M. In vitro antiviral activity of thirty-six plants from La Reunion Island. 2002;73: 346–350.
CrossRef - Dasuki S. 202 Benefits of Herbs, Grup Buku Karangkraf, Selangor, Malaysia. 2011;222–223.
- Wong S. P., Leong L. P., Koh J. H. W. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006;99:775–783.
CrossRef - Ng D. S. H., Rose L. C., Suhaimi H., Mohammad H., Rozaini M. Z. H., Taib M. Preliminary evaluation on the antibacterial activities of Citrus hystrix oil emulsions stabilized by Tween 80 and Span 80. J. Pharm. Pharm. Sci. 2011;3:209–211.
- Tanaka T. Misunderstanding with regard to citrus classification and nomenclature. Bul Univ Osaka Prefecture Ser. 1969;21:139–145.
- Nicolosi E., Deng Z., Gentile A., La Malfa S., Continella G., Tribulato E. Citrus phylogeny and genetic origin of important species as investigated by molecular markers. Theor Appl Genet. 2000;100:1155–1166.
CrossRef - Jabalpurwala F. A., Smoot J. M., Rouseff R. L. A comparison of citrus blossom volatiles. 2009;70:1428-1434.
CrossRe - Penjor T., Yamamoto M., Uehara M., Ide M., Matsumoto N., Matsumoto R., Nagano Y. Phylogenetic relationships of Citrus and its relatives based on matK gene sequences. PLoS ONE. 2013;8(4):e62574.
CrossRef - Liu X., Tang L., Wu H., Xi W., Yu J., Zhou Z. Development of DArT markers and evaluation of phylogenetic relationship of key Citrus Genet. Resour. Crop Evol. 2015;1307–1318.
- Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils: A review. Food Chem. Toxicol. 2008;46:446-475.
CrossRef - Teixeira B. S., Marques A., Ramos C., Serrano C., Matos O., Neng N. R., Nogueira J. M., Saraiva J. A., Nunes M. L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J Sci Food Agric. 2013;93(11):2707-2714.
CrossRef - Buchbauer G. The detailed analysis of essential oils leads to the understanding of their properties. Perfumer & Flavorist. 2000;25:64–67.
- Laohavechvanich., Muangnoi C., Butryee C., Kriengsinyos W. Protective effect of makrut lime leaf (Citrus hystrix) in HepG2 cells: implications for oxidative stress. Science Asia. 2009;36:112-117.
CrossRef - Norkaew O., Pitija K., Pripdeevech P., Sookwong P., Wongpornchai S. Supercritical fluid extraction and gas chromatographic-mass spectrometric analysis of terpenoids in fresh Kaffir lime leaf oil. Chiang Mai J. Sci. 2013;40:240–247.
- Jantan I., Ahmad A. S., Ahmad A. R., Ali N .A. M., Ayop N. Chemical composition of some Citrus oils from Malaysia. Essent. Oil Res. 1996;8:627–632.
CrossRef - Cheong M. W., Loke X. Q., Liu S. Q., Pramudya K., Curran P., Yu B. Characterization of volatile compounds and aroma profiles of Malaysian pomelo (Citrus grandis (L.) Osbeck) blossom and peel. Essent. Oil Res. 2011;23:34–44.
CrossRef - Mohd-Yusoff Z., Muhammad Z., Kasuan N., Rahiman M. H. F., Taib M. N. Effect of temperature on kaffir lime oil by using Hydro-diffusion steam distillation system. J. Anal. Sci. 2013;17:326–339.
- Muhammad Z., Yusoff Z. M., Nordin M. N. N., Kasuan N., Taib M. N., Rahiman M. H. F., Haiyee Z. A. Steam distillation with induction heating system: Analysis of Kaffir Lime oil compound and production yield at various temperatures. J. Anal. Sci. 2013;17:340–347.
- Kasuan N., Muhammad Z., Yusoff Z., Rahiman M. H. F., Taib M. N., Haiyee Z. A. Extraction of Citrus hystrix DC (Kaffir Lime) essential oil using automated steam distillation process: Analysis of volatile compounds. J. Anal. Sci. 2013;17:359–369.
- Waikedre J., Dugay A., Barrachina I., Herrenknecht C., Cabalion P., Fournet A. Chemical composition and antimicrobial activity of the essential oils from New Caledonian Citrus macroptera and Citrus hystrix. Biodivers. 2010;7:871–877.
CrossRef - Nor O.M. Volatile aroma compounds in Citrus hystrix. J. Trop. Agric. Food Sci. 1999;27:225–229.
- Loh F. S., Awang R. M., Omar D., Rahmani M. Insecticidal properties of Citrus hystrix DC leaves essential oil against Spodoptera litura fabricius. Med. Plants Res. 2011;5:3739–3744.
- Hongratanaworakit T., Buchbauer G. Chemical composition and stimulating effect of Citrus hystrix oil on humans. Flavour Fragr. J. 2007;22:443–449.
CrossRef - Vimol S., Chanwit T., Veena N., Nuntavan B., Kulkanya C., Siwimol P., Sirirat C., Somporn S. Antibacterial activity of essential oils from Citrus hystrix (makrut lime) against respiratory tract pathogens. Science Asia. 2012;38:212-217.
CrossRef - Aumeeruddy-Elalfi Z., Gurib-Fakim A., Mahomoodally M. F. Chemical composition, antimicrobial and antibiotic potentiating activity of essential oils from 10 tropical medicinal plants from Mauritius. Herbal Med. 2016;6:88-95.
CrossRef - Wungsintaweekul J., Sitthithaworn W., Putalun W., Pfeifhoffer H. W., Brantner A. Antimicrobial, antioxidant activities and chemical composition of selected Thai spices. Songklanakarin J. Sci. Technol. 2010;32(6):589-598.
- Raksakantong P., Siriamornpun S., Meeso N. Effect of drying methods on volatile compounds, fatty acids and antioxidant property of Thai kaffir lime (Citrus hystrix C.). Int. J. Food Sci. Technol. 2012;47:603–612.
CrossRef - Bravo L. Polyphenols: Chemistry dietary sources metabolism and nutritional significance. Rev. 1998;56:317–333.
CrossRef - Ambriz-Pérez D. L., Leyva-López N., Gutierrez-Grijalva E. P., Heredia J. B. Phenolic compounds: Natural alternative in inflammation treatment. A review. Cogent Food & Agric. 2016;2(1): 1131412. https://doi.org/10.1080/23311932.2015.1131412.
- Orak H. H. Total antioxidant activities, phenolics, anthocyanins, polyphenoloxidase activities of selected red grape cultivars and their correlations. Hort. 2007;111(5):235-241.
CrossRef - Miean, K.H., Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. Agric. Food Chem., 2001; 49: 3106−3112.
CrossRef - Ali Asgar, Md. Anti-diabetic potential of phenolic compounds: A review. J. Food Prop., 2013; 16: 91-103.
CrossRef - Patel, S.S., Goyal R.K. Cardioprotective effects of gallic acid in diabetes-induced myocardial dysfunction in rats. Pharmacognosy Res., 2011; 3(4): 239-245.
CrossRef - Jung, U.J., Lee, M.K., Jeong, K.S., Choi, M.S. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J Nutr., 2004; 134(10): 2499-2503.
- Aning, A., Wenny, I., Stefanus., Kevin, J., Chynthia, D.H., Adi, T.N. Optimization of phenolic compounds extraction from Averrhoa bilimbi and Citrus hystrix peel using statistical design of experiment. J Eng Appl Sci. 2016; 11(23): 13783-13789.
- Berhow, M., Tisserat, B., Kanes, K., Vandercook, C. Survey of phenolic compounds produced in U.S. Department of Agriculture; Agricultural Research Service, Technical Bulletin N°.1856, 1998; pp 158.
- Chan, S.W., Lee, C.Y., Yap, C.F., Wan, A.W.M., Ho, C.W. Optimisation of extraction conditions for phenolic compounds from limau purut (Citrus hystrix) peels. Food Res. J., 2009; 16: 203-213.
- Jamilah, B., Abdulkadir, G.M., Suhaila, M., Zaidul, Md.IS. Phenolics in Citrus hystrix leaves obtained using supercritical carbon dioxide extraction. Food Res. J., 2011; 18(3): 941–948.
- Murakami, A., Gao, G., Kim, O.K., Omura, M., Yano, M., Ito, C., Furukawa, H., Jiwajinda, S., Koshimizu, K., Ohigashi, H. Identification of coumarins from the fruit of Citrus hystrix DC as inhibitors of nitric oxide generation in mouse macrophage RAW 264.7 cells. J. Agric. Food Chem., 1999; 47(1):333-339.
CrossRef - Pereira, D.M., Valentão, P., Pereira, J.A., Andrade, P.B. Phenolics: From Chemistry to Biology., 2009; 14 (6): 2202-2211.
CrossRef - Murakami, A., Nakamura, Y., Koshimizu, K., Ohigashi, H. Glyceroglycolipids from Citrus hystrix, a traditional herb in Thailand, potently inhibit the tumor-promoting activity of 12-O-Tetradecanoylphorbol 13-Acetate in mouse skin. Agric. Food Chem., 1995; 43 (10): 2779–2783.
CrossRef - Panthong, K., Srisud, Y., Rukachaisirikul, V., Hutadilok-Towatana, N., Voravuthikunchai, S.P., Tewtrakul, S. Benzene, coumarin and quinolinone derivatives from roots of Citrus hystrix. Phytochemistry., 2013; 88: 79-84.
CrossRef - Chanthaphon, S., Chanthachum, S., Hongpattarakere, T. Antimicrobial activities of essential oils and crude extracts from tropical Citrus against food-related microorganisms. Songklanakarin J. Sci. Technol., 2008; 30: 125-131.
- Tuntipopipat, S., Muangnoi, C., Failla, M.L. Anti-inflammatory activities of extracts of Thai spices and herbs with lipopolysaccharide-activated RAW 264.7 murine macrophages. Med. Food., 2009; 12(6):1213–1220.
CrossRef - Abirami, A., Nagarani, G., Siddhuraju, P. In vitro antioxidant, anti-diabetic, cholinesterase and tyrosinase inhibitory potential of fresh juice from Citrus hystrix and maxima fruits, Food Sci. Human Well. 2014; 3: 16-25.
CrossRef - Abirami, A., Nagarani, G., Siddhuraju, P. Hepatoprotective effect of leaf extracts from Citrus hystrix and maxima against paracetamol induced liver injury in rats. Food Sci. Human Well. 2015; 4: 35-41.
CrossRef - Bayala, B., Bassole, I.H., Scifo, R., Gnoula, C., Morel, L., Lobaccaro, J.M.A., Simpore, J. Anticancer activity of essential oils and their chemical components – a review. J. Cancer Res. 2014; 4(6): 591–607.
- Manosroi, J., Dhumtanom, P., Manosroi, A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett., 2006; 235(8): 114-120.64.
- Chueahongthong, F., Ampasavate, C., Okonogi, S., Tima, S; Anuchapreeda, S. Cytotoxic effects of crude kaffir lime (Citrus hystrix, DC.) leaf fractional extracts on leukemic cell lines. J. Med. Plant Res., 2011; 5: 3097-3105.65.
- Wijayanti, N., Tunjung, W.A.S., Setyawati, Y. Cytotoxicity and apoptosis induction by kaffir lime leaves extract (Citrus hystrix DC.) in HeLa cells culture (human cervical cancer cell line). KnE Life Sciences, Int. Conf. on Biological Sciences., 2013; 631.
- Tunjung, W.A.S., Cinatl, J., Michaelis, M., Smales C.M. Anti-Cancer effect of Kaffir Lime (Citrus hystrix DC) leaf extract in cervical cancer and neuroblastoma cell lines. Procedia Chem., 2015; 14: 465-468.
CrossRef - Singhal, A.K., Naithani, V., Bangar, O.P. Medicinal plants with a potential to treat Alzheimer and associated symptoms. Int. J. Nutr. Pharmacol.Neurol. Dis., 2012; 2 (2): 84–91.
CrossRef - Youkwan, J., Sutthivaiyakit, S., Sutthivaiyakit, P. Citrusosides A-D and furanocoumarins with cholinesterase inhibitory activity from the fruit peels of Citrus hystrix. J. Nat. Prod., 2010; 73:1879-1883.
CrossRef - Chaiyana, W., Saeio, K., Hennink, W.E., Okonogi, S. Characterization of potent anticholinesterase plant oil based microemulsion. Int. J. Pharm., 2010; 401(30): 32-40.
CrossRef - Seeka, C., Sutthivaiyakit, P., Youkwan, J., Hertkorn, N., Harir, M., Schmitt-Kopplin, P., Sutthivaiyakit, S. Prenylfuranocoumarin–HMGA–flavonol glucoside conjugates and other constituents of the fruit peels of Citrus hystrix and their anticholinesterase activity. Phytochemistry., 2016; 127: 38-49.
CrossRef - Mya, M.M., Aye, Y.Y., Oo, A.W., Saxena, R.K. Effect of Citrus hystrix DC leaves ethanol extract on larvae of Aedes aegypti. J. Biol. Eng. Res. and Review., 2015; 2(2): 01-06.
- Jansen, C.C., Beebe, N.W. The dengue vector Aedes aegypti: what comes next. Microbes Infect., 2010; 12: 272-279.
CrossRef - Ansori, A.N.M., Supriyadi, A.P., Kartjito, M.V., Rizqi, F., Adrianto, H., Hamidah. Biolarvicidal effectivities of polar and non-polar extract fraction from Kaffir Lime (Citrus hystrix) leaf against 3rd instar larvae of Aedes aegypti. J Biol. Eng. Res. and Review., 2015; 2(2): 14-17.
- Nararak, J., Sathantriphop, S., Kongmee, M., Bangs, M.J., Chareonviriyaphap, T. Excito-repellency of Citrus hystrix DC leaf and peel essential oils against Aedes aegypti and Anopheles minimus (Diptera: Culicidae), vectors of human pathogens. J. Med. Entomol., 2016; 54(1):178-186.
CrossRef - Piyachaturawat, P., Glinsukon, T., Chanjarunee, A. Antifertility effect of Citrus hystrix DC. J. Ethnopharmacol., 1985; 13: 105-110.
CrossRef - Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci., 2009; 10(6): 2440–2475.
CrossRef - Aumeeruddy-Elalfi, Z., Gurib-Fakim, A., Mahomoodally, M.F. Kinetic studies of tyrosinase inhibitory activity of 19 essential oils extracted from endemic and exotic medicinal plants. South Afrcan J. Bot., 2016; 103: 89-94.
CrossRef - Putri, H., Nagadi, S., Larasati, Y.A., Wulandari, N., Hermawan, A. Cardioprotective and hepatoprotective effects of Citrus hystrix peels extract on rats model. Asian Pac. J. Trop. Biomed., 2013; 3:371-375.
CrossRef - Tisserand, R., Young, R: Essential oil safety: a guide for health care professionals. (Second Edition), Elsevier Health Sciences, Churchill Livingstone, St. Louis., 2014; pp. 784.

This work is licensed under a Creative Commons Attribution 4.0 International License.





