Manuscript accepted on : 10 July 2016
Published online on: --
Serum Level of Kisspeptin in Pregnant Women with Preeclampsia
Hassan Zabetian1, Reza Sahraei1, Hossein Hakimelahi1, Alireza Yusefi1*, Mohammad Sadegh Sanie1, Saeid Sobhanian1, Abdolreza Sotoodeh Jahromi1, Abdolali Sepidkar1, Mehrnoosh Maalhagh2, Abdolhossien Madani3, Farshid Kafilzadeh4 and Mohammad Kargar4
1Research Center for Non.communicable Diseases, Jahrom University of Medical Sciences, Jahrom, Iran.
2Reseach Committee, Shiraz University of Medical Sciences, Shiraz, Iran.
3Research Center for Social Determinants in Health Promotion, Hormozgan University of Medical Sciences, Bandarabbas, Iran.
4Department of biology, Jahrom branch, Islamic Azad University, Jahrom, Iran.
DOI : http://dx.doi.org/10.13005/bbra/2319
ABSTRACT: Preeclampsia is major causes of maternal and perinatal mortality and morbidity throughout the world. To diagnose preeclampsia, a large and growing number of biochemical markers have been tested as potential screening tests. Kisspeptin (KP) has recently seems as putative biomarker for preeclampsia. Therefore, the aim of study was to evaluate the sensitivity, specificity and diagnostic accuracy of KP in the second-trimester of pregnancy. This diagnostic study was conducted on 430 women in second-trimester of pregnancy. Serum concentration of KP was measured by standard sandwich enzyme-linked immune-Sorbent assay method. Sensitivity, specificity, accuracy, Likelihood ratio of a Positive and negative Test, positive and negative predictive values of the KP were calculated. Also, receiver operating characteristic (ROC) curves were used.KP mean serum level were 75.56 ± 91.76 (ng/ml) and 34.68 ± 32.86 (ng/ml) in 430 participants, respectively. Serum level of KP for diagnosis of preeclampsia in the second trimester showed that the sensitivity and specificity of the test were 33.33% and 95.36%, respectively. Diagnostic Accuracy of test was 93.27%. Positive Predictive Value and Negative Predictive Value were 20% and 97.62%, respectively. Area in ROC curve shows that possibility of diagnosing preeclampsia with KP serum level is 64.3%. To diagnose the preeclampsia in early pregnancy, KP level is a promising biomarker but regarding to inadequate test sensitivity, it cannot be recommended in screening.
KEYWORDS: kisspeptin; preeclampsia; pregnant women; screening
Download this article as:| Copy the following to cite this article: Zabetian H, Sahraei R, Hakimelahi H, Yusefi A, Sanie M. S, Sobhanian S, Jahromi A. S, Sepidkar A, Maalhagh M, Madani A, Kafilzadeh F, Kargar M. Serum Level of Kisspeptin in Pregnant Women with Preeclampsia. Biosci Biotech Res Asia 2016;13(3). |
| Copy the following to cite this URL: Zabetian H, Sahraei R, Hakimelahi H, Yusefi A, Sanie M. S, Sobhanian S, Jahromi A. S, Sepidkar A, Maalhagh M, Madani A, Kafilzadeh F, Kargar M. Serum Level of Kisspeptin in Pregnant Women with Preeclampsia. Biosci Biotech Res Asia 2016;13(3). Available from: https://www.biotech-asia.org/?p=15745 |
Introduction
Preeclampsia is one of most important human pregnancy-specific complication that affects approximately 3-5% of pregnant women and also is major causes of maternal and perinatal mortality and morbidity throughout the world (Young, Levine et al. 2010). Generally, the preeclampsia defined as new onset of hypertension (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg) and proteinuria (≥ 300 mg/24 hours or significant increase from baseline, onset of symptoms after 20 weeks gestation with remission by 6–12 weeks postpartum) by the International Journal of Women’s Health guidelines (Fabry, Richart et al. 2010). Although the exact mechanism of preeclampsia remains elusive, but it is characterized by increased apoptosis of trophoblastic cells, impaired trophoblastic invasion, abnormal placental function, placental ischemia and defective maternal spiral artery modification (Shamshirsaz, Paidas et al. 2011).
To diagnose preeclampsia, a large and growing number of biochemical markers have been tested as potential screening tests, however, none of them is a reliable markers(4), Certainly, in result of inadequate test sensitivity and specificity, none of them have confirmed as a routine clinical tests (Burney, Schust et al.). But a number of factors have been implicated in trophoblast invasion that one of them is metastin (which is encoded by the KiSS-1 gene)(5), therefore, kisspeptin (KP) has recently seems as putative biomarker for preeclampsia (Smets, Deurloo et al. 2008, Vazquez-Alaniz, Galaviz-Hernandez et al. 2011).
The KiSS-1 gene encodes a 145 amino acid primary peptide that cleaved to a 54 amino acid peptide, metastin, also known as KP or Kp-54 (Bilban, Ghaffari-Tabrizi et al. 2004). KP is bound to the G-protein-coupled receptor GPR54 (Mead, Maguire et al. 2007). Both KP and GPR54 are expressed in the placenta of normal pregnant women (Park, Lee et al. 2012). Some recognized roles of KP include metastasis suppressor, assistance in reproductive function through onset of puberty due to stimulating hypothalamic Gonadotropin-Releasing Hormone (GnRH) release(10) and their roles in placentation, regulation of reproduction, pregnancy and cardiovascular function (Dungan, Clifton et al. 2006). Also the metastin/GPR54 produced by first-trimester trophoblast cells, has an important role in controlling of migratory features and blocking trophoblastic invasion (Baba, Kang et al. 2014).
Qiao C, et al. results demonstrated the trophoblast expression level of KiSS-1 in preeclamptic women was higher in comparison with normal controls. Regarding to the role of metastin/GRP54 system in inhibiting trophoblast invasion(12), As well as, proven link between preeclampsia with trophoblast invasion(3), they supposed that an abnormal metastin/GRP54 system may be involved in the pathogenesis of early-onset preeclampsia (Qiao, Wang et al. 2012).
Difficulties to use of KP as a biomarker include its unclear secretion profile in healthy pregnancy and lack of standardization of KP assays. Therefore the aims of this study were to evaluate the sensitivity, specificity and diagnostic accuracy of KP in the second-trimester of pregnancy and determine its predictive value for identifying women at risk of developing preeclampsia.
Material and Method
This diagnostic study was conducted to evaluate sensitivity and specificity of KP serum level in order to predict preeclampsia compared with gold standard test (hypertension or proteinuria). Subjects were recruited from the women who referred to Obstetricians clinic of Jahrom university of medical sciences, Jahrom, Iran (September – December 2014). Exclusion criteria were history of preeclampsia, gestational Diabetes mellitus (GDM), abnormal Body Mass Index (BMI), intrauterine growth restriction (IUGR), inflammatory or infective disorders and heart disease, treatment with aspirin, nonsteroidal anti-inflammatory drugs, antibiotics, Lipid-lowering or antihypertensive drugs, systemic disease like hypertension, diabetes mellitus or thyroid diseases. According to the inclusion and exclusion criteria 430 women in second-trimester of pregnancy were randomly selected. Before sampling, Written Informed consents were obtained from participants. This study conforms to the declaration of Helsinki regarding research involving human subjects and approved by the ethics committee of Jahrom University of Medical Sciences.
Serum samples were collected at second-trimester (22th week gestational age). Samples were immediately centrifuged (2500 g for 15 min); serum samples were stored at −70 ◦C until KP quantitative assay.
Body mass index (BMI) was calculated as weight divided by height2, (kg/m2). The blood pressure measurements taken after 5 minutes seated at rest using an automatic sphygmomanometer. Midstream urine sample and 24-hour urine collection was obtained from each participant women were carefully matched for age, gestational age, and BMI.
Patients who diagnosed as preeclampsia based on systolic blood pressure (BP) ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg or proteinuria with a urine dipstick of ≥ 1+ or ≥ 300 mg per 24 hours, after 20 weeks’ gestation observed. Serum level of KP measured in healthy pregnant participants and those who developed with preeclampsia at 22th week and compared together. Serum concentration of KP was measured in the second-trimester of pregnancy (22th week) by standard sandwich enzyme-linked immune-Sorbent assay technology (Chongqing Biospes Co., Ltd). Lower range of KP detection was 5 pg/ml.
Statistical analysis
Sensitivity, specificity, accuracy, Likelihood ratio of a Positive and negative Test, positive predictive values (PPV) and negative predictive values (NPV) of the KP were calculated. Receiver operating characteristic (ROC) curves were used to evaluate the KP serum level in screening of preeclampsia in the second-trimester of pregnancy. The statistical analyses were performed using the SPSS software version 12.0 for Windows. P-values <0·05 was assumed statistically significant.
Results
Continuous variables reported as mean and standard deviation (Mean ± SD). The mean age of participants was 28.7 years old. Frequency of women who diagnosed as preeclampsia patients in the second trimester was 13 individuals (3.1%).
Paraclinical results (urine sample and blood pressure) showed that 416 pregnant women were normotensive (without any signs of gestational complications or fetal distress) and serum level of KP was in normal range during pregnancy. Five women developed preeclampsia based on blood pressure and urine sample but the serum level of KP was not in the range of preeclampsia women. Twenty five women had elevated serum level of KP but they had no sign or symptoms of preeclampsia (table 1).
Table 1: Frequency of elevated serum level of KP in pregnant women with and without preeclampsia
| PRE ECLAMPSIA | |||
| KISSPEPTIN | Positive | Negative | Total |
| Positive | 0 | 25 | 25 |
| Negative | 5 | 416 | 421 |
| Total | 5 | 441 | 446 |
KP mean serum level in 430 participants were 75.56 ± 91.76 ng/ml.
Serum level of KP in women with preeclampsia for diagnosis of preeclampsia in the second trimester showed that the sensitivity and specificity of the test were 33.33% and 95.36%, respectively. Diagnostic accuracy of test was 93.27%. PPV and NPV were 20% and 97.62%, respectively. ROC curve determines low sensitivity of serum level of KP in the diagnosis of preeclampsia (sig: 0.059). Area in ROC curve shows that possibility of diagnosing preeclampsia with KP serum level is 64.3% (Fig-1).
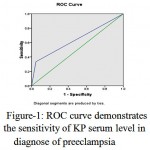 |
Figure 1: ROC curve demonstrates the sensitivity of KP serum level in diagnose of preeclampsia |
Discussion
To prevent adverse consequences of Preeclampsia in mother and neonate, early detection of Preeclampsia is so crucial (Burney, Schust et al.). KP has been proposed as a novel biomarker for identifying Preeclampsia (Baba, Kang et al. 2014) but inadequate data exist to demonstrate its accuracy to use in routine screening tests(6, 16). Trophoblasts and uterine cells interactions are regulated by mechanisms that are largely unknown. These regulations are carried out by different factors such as cytokines and hormones produced by the fetal and maternal tissues (Knöfler and Pollheimer 2012).
There is some contradiction between studies about increase or decrease of KP serum levels in preeclamptic patients. Cartwright & Williams(18) found reduced expression of KiSS-1 in preeclamptic patients compared with control group at both protein and mRNA levels. They also survayed first-trimester placentas with high and normal uterine artery and demonstrated reduced KiSS-1 level in the high resistance group (Cartwright and Williams 2012). In another study which cinducted by Cetkovic A et al. it was found same result in the second and third trimesters (Ćetković, Miljic et al. 2012). In contrast to the mentioned studies, Zhang H et al.(19) and Qiao C et al.(15) demonstrated a significant increase in KiSS-1 expression both at the RNA and protein levels in the placenta of preeclampsia (Zhang, Long et al. 2011, Qiao, Wang et al. 2012). Furthermore, Vazquez-Alaniz F et al(20) observed same results that higher expression for KiSS-1 exist in preeclamptic women against control group (Vazquez-Alaniz, Galaviz-Hernandez et al. 2011).
This discrepancy is maybe due to the low number of subjects participated in Cartwright & Williams study, (10 or 6 in each group) and using Doppler to predict preeclampsia is seen to have a high false-negative rate (Cartwright and Williams 2012). Unlike previous studies, the results of our study indicated increasing maternal serum KP during the second trimester of pregnancy.
In present study serum level of KP increased in all pregnant women and agreed the results of previous studies (Zhang, Long et al. 2011, Qiao, Wang et al. 2012). Otherwise, serum level of KP in pregnant women who diagnosed as preeclampsia in 22th week were less than serum level that reported in studies done on preeclampsia patients in third trimester (not in the range to diagnose preeclampsia).
In a study that was conducted on Chinese women, they discussed that metastin is expressed in the placenta of normal pregnancy and regulate trophoblast invasion through inhibiting migration of trophoblast cells. Further, they showed that KiSS-1 expression is increased in placenta of intrauterine death in comparison with normal newborns of early-preeclampsia and late-preeclampsia (Qiao, Wang et al. 2012). However Cetkovic A shwed low KP levels were associated with adverse perinatal outcome in the third-trimester in patients with preeclampsia and placental dysfunction (Ćetković, Miljic et al. 2012).
Results of Zhang H et al.(19) study showed during placental development, KiSS-1 gene has an important role in inhibiting trophoblast invasion (Zhang, Long et al. 2011) and according to F et al. data(20), it could be concluded that high levels of KP in preeclampsia patients, represents KP relationship with its role as trophoblastic invasiveness inhibitor (Vazquez-Alaniz, Galaviz-Hernandez et al. 2011).
Nijher et al. reported no association between KP levels in the third-trimester of pregnancy and blood pressure (Nijher, Chaudhri et al. 2010). Furthermore, they suggested that high concentration of serum KP do not play a key role in pathogenesis of hypertensive diseases of pregnancy. However, Logie J et al. observed negative associations between KP levels in early pregnancy and blood pressure in mid-late pregnancy (Logie, Denison et al. 2012). However, it cannot be determined whether change in metastin levels is a cause or a consequence of disease.
Further studies with more numbers of patients are needed to obtain more information regarding this topic.
Comparing predictive values of each test with other tests is valuable. In order to screen, the more positive predictive value of a test, the more value of the test in ruling in the considered disease. Also, the less positive predictive value of a test, the more value of the test in ruling out the considered disease(23).
The high negative predictive value of KP demonstrates that the test is effective in ruling out the preeclampsia, while relatively low positive predictive value of it demonstrates its ineffectiveness to rule in the diagnosis.
In fact, relatively low KP sensitivity; as a diagnostic test for preeclampsia; indicates that a significant patients proportion will be undiagnosed.
In our study, the largest area under the ROC curve is 0.643, indicating that is statistically admissible as the screening tool.
In a study that conducted by Logie JJ et al, to predict of preeclampsia, 16-week plasma KP, area under the receiver-operator characteristic curve was 0·80 (P < 0·01), positive and negative likelihood ratios were 3·0 and 0·2, and test sensitivity and specificity were 85·7 and 71·4%, respectively (Logie, Denison et al. 2012). Also based on our result despite of KP have high specificity and negative predictive value but have low sensitivity and positive predictive value and about ROC cure, significant correlation was not seen.
Therefore, according to our findings and previous studies, to diagnose the preeclampsia in early pregnancy, KP level is a promising biomarker but regarding to inadequate test sensitivity, it cannot be recommended in screening. Improving the sensitivity and positive predictive value of KP, may be used it as a potential marker to predict adverse perinatal outcome in pregnancies with placental dysfunction. Moreover, further studies with more patients in different weeks of pregnancy could be useful.
References
- Baba, T., H. S. Kang, Y. Hosoe, B. Kharma, K. Abiko, N. Matsumura, J. Hamanishi, K. Yamaguchi, Y. Yoshioka and M. Koshiyama (2014). “Menstrual cyclic change of metastin/GPR54 in endometrium.” Medical molecular morphology: 1-9.
- Bilban, M., N. Ghaffari-Tabrizi, E. Hintermann, S. Bauer, S. Molzer, C. Zoratti, R. Malli, A. Sharabi, U. Hiden and W. Graier (2004). “Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts.” Journal of Cell Science 117(8): 1319-1328.
- Burney, R. O., D. J. Schust and M. W. Yao “Berek & Novak’s Gynecology 14th Edition.”
- Cartwright, J. E. and P. J. Williams (2012). “Altered placental expression of kisspeptin and its receptor in pre-eclampsia.” Journal of Endocrinology 214(1): 79-85.
- Ćetković, A., D. Miljic, A. Ljubić, M. Patterson, M. Ghatei, J. Stamenković, M. Nikolic-Djurovic, S. Pekic, M. Doknic and A. Glišić (2012). “Plasma kisspeptin levels in pregnancies with diabetes and hypertensive disease as a potential marker of placental dysfunction and adverse perinatal outcome.” Endocrine research 37(2): 78-88.
- Dungan, H. M., D. K. Clifton and R. A. Steiner (2006). “Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion.” Endocrinology 147(3): 1154-1158.
- Fabry, I., T. Richart, X. Cheng, L. Van Bortel and J. A. Staessen (2010). “Diagnosis and treatment of hypertensive disorders during pregnancy.” Acta Clinica Belgica 65(4): 229-236.
- Knöfler, M. and J. Pollheimer (2012). “IFPA Award in Placentology lecture: molecular regulation of human trophoblast invasion.” Placenta 33: S55-S62.
- Logie, J. J., F. C. Denison, S. C. Riley, T. Ramaesh, S. Forbes, J. E. Norman and R. M. Reynolds (2012). “Evaluation of kisspeptin levels in obese pregnancy as a biomarker for pre‐eclampsia.” Clinical endocrinology 76(6): 887-893.
- Mead, E. J., J. J. Maguire, R. E. Kuc and A. P. Davenport (2007). “Kisspeptins are novel potent vasoconstrictors in humans, with a discrete localization of their receptor, G protein-coupled receptor 54, to atherosclerosis-prone vessels.” Endocrinology 148(1): 140-147.
- Nijher, G. M., O. B. Chaudhri, R. Ramachandran, K. G. Murphy, S. E. Zac‐Varghese, A. Fowler, K. Chinthapalli, M. Patterson, E. L. Thompson and C. Williamson (2010). “The effects of kisspeptin‐54 on blood pressure in humans and plasma kisspeptin concentrations in hypertensive diseases of pregnancy.” British journal of clinical pharmacology 70(5): 674-681.
- Park, D. W., S. K. Lee, S. R. Hong, A. R. Han, J. Kwak‐Kim and K. M. Yang (2012). “Expression of Kisspeptin and its receptor GPR54 in the first trimester trophoblast of women with recurrent pregnancy loss.” American Journal of Reproductive Immunology 67(2): 132-139.
- Qiao, C., C. Wang, J. Zhao, C. Liu and T. Shang (2012). “Elevated expression of KiSS-1 in placenta of Chinese women with early-onset preeclampsia.”
- Shamshirsaz, A. A., M. Paidas and G. Krikun (2011). “Preeclampsia, hypoxia, thrombosis, and inflammation.” Journal of pregnancy 2012.
- Smets, E. M., K. L. Deurloo, A. T. Go, J. M. van Vugt, M. A. Blankenstein and C. Oudejans (2008). “Decreased plasma levels of metastin in early pregnancy are associated with small for gestational age neonates.” Prenatal diagnosis 28(4): 299-303.
- Vazquez-Alaniz, F., C. Galaviz-Hernandez, L. A. Marchat, J. M. Salas-Pacheco, I. Chairez-Hernandez, J. J. Guijarro-Bustillos and A. Mireles-Ordaz (2011). “Comparative expression profiles for KiSS-1 and REN genes in preeclamptic and healthy placental tissues.” European Journal of Obstetrics & Gynecology and Reproductive Biology 159(1): 67-71.
- Young, B. C., R. J. Levine and S. A. Karumanchi (2010). “Pathogenesis of preeclampsia.” Annual Review of Pathological Mechanical Disease 5: 173-192.
- Zhang, H., Q. Long, L. Ling, A. Gao, H. Li and Q. Lin (2011). “Elevated expression of KiSS-1 in placenta of preeclampsia and its effect on trophoblast.” Reproductive biology 11(2): 99-115.

This work is licensed under a Creative Commons Attribution 4.0 International License.





