Manuscript accepted on : 11 May 2016
Published online on: --
Indrakshi Paul1, K. A. Athmaselvi1 and P. Geetha2
1Department of Food Process Engineering, SRM University, Tamil Nadu, India.
2College of Food and Dairy Technology, TANUVAS, Koduvalli, Tamil Nadu, India.
Corresponding Author E-mail: Indrakshipaul1701@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2155
ABSTRACT: Extensive research on lignan has revealed numerous nutritional and health benefits due primarily to its nutrients content. Flaxseed is the richest source of lignan, so flax lignan was used for the investigation. The objective of this study was to evaluate the effect of 3.75 % flax lignan supplementation on the physicochemical, microbiological and sensorial properties of mishti dahi during storage. Mishti dahi with 3.75 % flax lignan was prepared by replacing sugar with 10 % honey. There were no such differences in pH, moisture, acidity, total solids and whey syneresis values between the flax lignan incorporated mishti dahi and control. With respect to sensorial attributes, there is also no such differences observed between the two samples during storage. Microbiologically, it gave the shelf life of 20 days for lignan supplemented mishti dahi as compared to control which showed a shelf life of 15 days during its storage studies. Lignan incorporated mishti dahi was later investigated for its antidiabetic activity by comparing alpha amylase activity at a concentration of 100µg/ml and was found to be 48.41% against acarbose (standard antidiabetic drug). Therefore, the developed product could serve as a potent antidiabetic and hence can serve humanity with its health benefits.
KEYWORDS: Flaxseed; lignan; functional food; antidiabetic; health benefits
Download this article as:| Copy the following to cite this article: Paul I, Athmaselvi K. A, Geetha P. Physicochemical, Sensorial and Microbiological Properties of Mishti Dahi Supplemented With Flax Lignan During Storage And its Antidiabetic Activity. Biosci Biotech Res Asia 2016;13(2) |
| Copy the following to cite this URL: Paul I, Athmaselvi K. A, Geetha P. Physicochemical, Sensorial and Microbiological Properties of Mishti Dahi Supplemented With Flax Lignan During Storage And its Antidiabetic Activity. Biosci Biotech Res Asia 2016;13(2). Available from: https://www.biotech-asia.org/?p=13866 |
Introduction
The flaxseed’s latin name is Linum usitatissimum, which means “very useful”. Flaxseed has many biologically active components that has a potential health benefits and that’s why flaxseed has been the focus of increased interest in the field of diet and disease research. Hence, each and every part of the flaxseed plant is utilized commercially, either directly or after processing. Flaxseed has a rich contents of α-linolenic acid (ALA), lignans, and fiber and thus emerging as a functional food ingredient. It can contribute to the reduction of several diseases such as arteriosclerosis, diabetes mellitus, and cancer.
From the industrial point of view, flaxseed is one of the most important oilseed crops and also it has a role in food, feed, and fiber purposes. Every part of the flaxseed yield something or the other like its seed provides oil which is rich in lignans, omega-3, digestible proteins and stem yields good quality fiber having high durability and strength. The. Lignans are anti-carcinogenic and antidiabetic compounds.
Many people get confused between lignins and lignans but they are very different, so should not be confused with each other. Lignans are stereospecific dimers of cinnamic alcohols i.e.; monolignols bonded at carbon 8 (C8-C8) as depicted in the figure1. Usually plant lignans occur free or bound to sugars in the plant. Monolignols are derived from hydroxycinnamic acids, they are either dimerized to lignans in the cell or in the cell wall they are polymerized into larger lignin structures. These structurally diverse group of compounds are involved in plant defense mechanism (as antioxidants, phytoalexins, biocides, etc.), providing protection against diseases and pests.
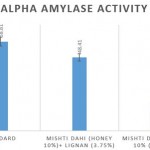 |
Figure 1: Structure of Secoisolariciresinol Diglycoside (SDG) |
The richest dietary source of lignans are flaxseeds, in which glycosides of secoisolariciresinol (SECO) i.e.; secoisolariciresinol diglucoside (SDG) and matairesinol (MAT) are the major components. They also have traces of pinoresinol, lariciresinol and isolariciresinol along with themselves [1]. Diglucosides of pinoresinol, SECO and syringaresinol are common. In flaxseed, SECO is present as a diglucoside and is part of an ester-linked complex or oligomer containing 3-hydroxyl-3-methylglutaric acid, a number of cinnamic acid glycosides and the flavonoid herbacetin.
Flax lignan have many potential health benefits such as atherosclerosis, in reducing cardiovascular disease, diabetes, arthritis, osteoporosis, cancer, autoimmune, neurological disorders and lowering the blood cholesterol. Flax protein also helps in the treatment of heart disease and in supporting the normal functioning of immune system. Flax lignans are biologically active so they have anticancer and antiviral effects which influence gene expression (activation) and may also protect against estrogen-related diseases such as menopausal pain and osteoporosis. The main flax lignan SDG is an antioxidant and also the antioxidant action of SECO and enterodiol is greater than that of vitamin E. After ingestion, plant lignans are converted by the human intestinal bacteria to the enterodiol and enterolactone and hence known as mammalian lignans. These metabolites are considered to be responsible for the biological effects in humans [2].
Dahi, indian curd, is a well-known fermented milk product consumed by large population throughout the country, either as a part of their daily diet or as a refreshing food. Curd is one of the most popular fermented dairy products, which is produced and consumed in rural areas of many countries in the world. It is thick, creamy, smooth, and of uniform appearance and usually has a white colour. It is semi-solid and has a pleasant odour, flavour and taste [3]. One of the most important fermented milk which is gaining popularity is Mishti Dahi (sweet curd). The viscosity of the milk is the vital characteristic of mishti dahi with regards to overall satisfaction. Dahi has probiotics which will help in maintaining gut bacteria and thus helps in proper functioning of digestive system. So dahi will be the best carrier for lignan to be supplemented in human body and by doing this health benefits can be doubled up.
As the only available natural sweetener honey from the ancient times, was an important food for Homo sapiens. The first written reference with respect to honey, dating back to 2100-2000 BC, mentions honey’s use as a drug and an ointment. In the ancient period, honey has been used for both medical and nutritional purposes. The belief that honey is a drug, a nutrient and an ointment has been carried into our days. An alternative medicine branch, called apitherapy, has developed in recent years in which offering treatments based on honey and also uses other bee products against many diseases. The knowledge on this subject is compiled in various books or on relevant web pages such as www.apitherapy.org, www.apitherapy.com for the human help. Beyond carbohydrates, honey contains numerous compounds such as, amino acids, organic acids, proteins, minerals, vitamins, polyphenols and aroma compounds. However, its importance with respect to nutrition that lies in the manifold physiological effects. It should be noted that the composition of honey depends greatly on the botanical origin, a fact that has been seldom considered in the physiological and nutritional studies. Honey has varied functionality depending on its botanical origin. Honey shows antiviral, antimicrobial and antiparasitic activity as well i.e.; inhibits the growth of micro-organisms and fungi. Also shows antiinflammatory, antioxidant, antimutagenic, anticancer and immunosuppressive activities. Different nutritional studies have confirmed various effects after honey ingestion, e.g. enhanced gastroenterological and cardiovascular health. Hence honey will be the best option for making mishti dahi due its health benefits.
Materials and Methods
Collection of Material
The flaxseed sample and fresh milk was collected from the local market (Rogers supermart), guduvenchery, Chennai, Tamilnadu for preparing flax lignan supplemented mishti dahi. Also Dabur honey was purchased from the local market and used as sweetner in making of Mishti Dahi.
Incorporation of flax Lignan into Mishti Dahi to develop a functional food
Functional food is developed by incorporating flax lignan into the mishti dahi in which honey is used as sweetner
General methodology for Preparing Mishti Dahi with honey as sweetner
First of all, milk was boiled and cooled down to 40 ͦ C. 40 ml of milk was taken into a small cup. To that 10% honey (Dabur honey) was added in the milk and mixed well. 2g of starter culture (premade curd of Hutsan purchased from local market) was added. Again mixed well and cup was closed with the airtight lid. Kept undisturbed for fermentation at room temperature for 6- 8 hours. Net weight of prepared mishit dahi with honey as sweetner was in the range of 58 gm and this was served as a control for the experiment.
Preparation of mishti dahi (honey as sweetner) with 3.75 % of lignan
Same as above but along with honey, 3.75% of lignan was added to the milk Net weight of the prepared mishit dahi was in the range of 58 gm. Here flax lignan was extracted from defatted flaxseeds using solvent system of ethanol and distilled water. Later presence of lignan was confirmed using phytochemical analysis.
Evaluation of sensorial property
It involves the measurement, quantification, and interpretation of the sensory characteristics of foods and consumer product through the use of human subjects acting as judges. The organoleptic attributes of the control and test samples was evaluated by the expert panel of judges on 9-point hedonic scale where a score of 9 represented “like extremely” and score of 1 represented “dislike extremely” [4]. Scores were given by the subjects based on the colour and appearance, taste, body and texture and overall acceptability.
Determination of physicochemical property
The developed products were analyzed for physico-chemical parameters, namely, pH, moisture content, titratable acidity, color.
pH
The electrode assembly of a digital ORLAB pH meter was calibrated against standard buffer of pH 7.0 and 4.0 (Qualigens Fine Chemicals). Then the pH of the samples was determined using the calibrated electronic pH meter at 30 ͦC.
Titratable acidity: The titratable acidity in the samples were estimated by calculation as outlined in IDF (1991).
Total soluble solids
Total soluble solids of the test sample and control were measured using refractrometer and were expressed in degree brix
Moisture content
The moisture contents of prepared mishit dahi and control sample were determined by the method as detailed in IS: SP: 18, Part XI, 1981.
Syneresis
Syneresis (spontaneous whey separation) was determined according to the procedure described by [5].
Color Analysis
The color value (L*, a*, b*) of the mishit dahi samples were measured using hunter lab color flex meter (Hunter Associates Laboratory Inc., Reston, Virgina (USA). The color was measured using Hunter lab scale at 100 observer and D65 illuminant. The information given by L, a and b is generally expressed as total color of the product. The axis –a* to + a* represents from green to red. The axis –b* to b* represents from blue to yellow and luminance is vertical axis from black (L = 0) to white (L = 100). The instrument was standardized each time with white and black ceramic tiles. The experiment was repeated two times and the mean L*, a* and b* values were recorded for all the samples.
Microbiological Analysis
Standard plate count
The enumeration of standard plate count was carried out as per procedure given in IS: (SP: 18, Part IX, 1981). The sterilized agar medium (Milk Agar) was melted and was kept at 48-50 ͦ C. The plates containing media and the sample dilutions were inverted and incubated at 37 ͦ C for 48 hours. Thereafter, count was taken and expressed as logarithmic value of colony forming units.
Yeast & Mould count
The enumeration of yeast and mould count was carried out as per procedure given in IS: (SP: 18, Part IX, 1981). Yeast and mould count was determined by plating 1:10, 1:100 and 1:1000 dilutions using potato dextrose agar. The count was taken after 5 days of incubation at 25-30 ͦC and expressed as logarithmic value of colony forming units per gram of sample.
Coliform count
The coliform group of bacteria comprises all aerobic and facultatively anaerobic, gram-negative non spore forming rods capable of fermenting lactose with the production of acid and gas at 37°C within 48 hours. Typically, these organisms are classified in the genera Escherichia and Enterobacter but, in addition, a few lactose-fermenting species of other genera are included in the group. In proportion to the numbers present, the existence of any of these types in dairy products is suggestive of unsanitary conditions or practices during production, processing or storage. The maximum standards for number of coliforms have until now been set at a maximum of 10 per milliliter of gram in pasteurized milk and milk products; however, numbers in pasteurized dairy products should be less than 1 ml., as is the case with up to 90% of samples, if packaging procedures are correct. Coliform count was determined by plating sample dilutions using violet red bile agar. The count was taken after 48 hours of incubation at 37 ͦC and expressed as logarithmic value of colony forming units per gram of sample.
Determination of antidiabetic property in the developed mishti dahi
α-amylase inhibition assay/Antidiabetic activity: The effect of sample on α-amylase activity can be studied using an enzyme-starch system. Samples were mixed by stirring with 25 mL of 4% potato starch in a beaker; 100 mg of α-amylase is added to the starch solution, stirred vigorously, and incubated at 37°C for 60 minutes. After the incubation period 0.1 M NaOH is added, to terminate enzyme activity. The mixture is centrifuged (3000 rpm; 15 minutes) and the glucose content in the supernatant was determined.
% Inhibition = [(Acontrol–A sample)/Acontrol] × 100 (1)
Results and discussion
Storage Studies
Changes in various parameters of mishti dahi has been observed when stored at 4 ͦ C.
Changes in physico-chemical properties of mishti dahi
The pH of Mishti Dahi supplemented with lignan decreased from 4.35 ± 0.01 to 3.92 ±0.01 whereas there is a more drop in the pH of control mishti dahi which were tabulated in the table2 below. Hence, the decrease in pH was accompanied by an increase in acidic taste of mishti dahi samples resulting in decreased taste scores. TA increased from 0.79 ± 0.04 to 1.46 ± 0.02 in 20 days, shown in table 2. The TA values were similar to the results obtained by O’Neil et al. [6] where it has been observed that there was an increase in TA values during storage.
Total soluble solid (Brix), it showed that the mishti dahi with lignan had significantly lower soluble solid percentage as compared to control mishti dahi. It also suggested that the lignan addition into mishti dahi changed the soluble solid (Brix) slightly. As clearly shown from the table that, the total soluble solid (Brix) of mishti dahi with lignan has not significantly lower as compared to the control mishti dahi shown in table 2 and table 1 respectively. This might be due to the liquid nature of lignan added to the mishti dahi.
Table 1: Effect of Storage On Physico-Chemical Properties of Product Taken as Control
| Storage days | pH | Acidity (%) | Total Solid ( ͦbrix) | Moisture Content (%) | Whey syneresis (ml/100ml) |
| 0th day | 4.2 ± 0.01 | 0.8± 0.02 | 26 ± 0.3 | 87.01± 0.5 | 15.35 ± 0.1 |
| 5th day | 4.17± 0.02 | 0.84± 0.04 | 26.1± 0.4 | 86.94± 0.6 | 16.81 ± 0.3 |
| 10thday | 4.09± 0.03 | 0.89± 0.02 | 25.7± 0.1 | 86.87± 0.1 | 17.65 ± 0.4 |
| 15thday | 4.01± 0.01 | 1.2± 0.06 | 25.3± 0.4 | 86.77± 0.3 | 19.19 ± 0.5 |
| 20thday | 3.87± 0.02 | 1.46± 0.04 | 25.1± 0.3 | 86.63± 0.3 | 21.11 ± 0.5 |
Moisture content of the samples i.e.; developed product and control both were showing a loss in moisture content but throughout its storage period the overall moisture loss from the samples were not much significant. Loss of moisture may be attributed to its chemical reaction in and around the atmosphere throughout its storage inside the air tight container. Syneresis were increased in both of the samples i.e.; control and developed product during storage. Mishti dahi containing lignan had not much significantly lower syneresis as compared to the mishti dahi without lignan. The syneresis values were similar to the results obtained by Farooq and Haque.
Table 2: Effect of Storage On Physico- Chemical Properties of Developed Product
| Storage days | pH | Acidity (%) | Total Solid ( ͦ brix) | Moisture Content (%) | Whey Syneresis (ml/100ml) |
| 0th day | 4.35± 0.01 | 0.79± 0.04 | 25.6± 0.2 | 87.05± 0.1 | 14 ± 0.1 |
| 5thday | 4.3± 0.04 | 0.82± 0.01 | 25.4± 0.4 | 86.99± 0.3 | 16.75 ± 0.2 |
| 10thday | 4.22± 0.03 | 0.91± 0.01 | 25.2± 0.3 | 86.89± 0.4 | 17.25 ±0.3 |
| 15thday | 4.12± 0.09 | 1.02± 0.03 | 24.9± 0.3 | 86.80± 0.5 | 18.65 ± 0.2 |
| 20thday | 3.92± 0.01 | 1.46± 0.02 | 24.7± 0.1 | 86.67± 0.1 | 19.41 ± 0.3 |
Changes in microbiological properties
SPC of Mishti Dahi was showed in Table 3. It can be seen clearly from table that standard plate count significantly increased from 5.11 ± 0.06 to 5.69 ± 0.05 (log cfu/g). However, lab count of both mishti dahi with lignan and control mishti dahi were significantly increased during storage. This results were similar to [7] and they found that total LAB count increased from 5.47 to 5.70 (log cfu/g) during storage. Most LAB bacteria are probiotics and have various health benefits to human being. Growth capacity of probiotics are important for the success of fermentation processes. In addition to this, the viability of these bacteria is crucial for the quality and stability of organic products. Microbiological quality control and assurance of fermented milk products is of primary importance in ensuring food safety and quality as well as conforming to the existing standards and/or regulations in the country of sale. The international standards describe that at the time of purchase the fermented products with health benefits must contain a minimum of 106 viable probiotic bacteria per gram [8].
Table 3: Standard plate count, coliform count, yeast and mould count of optimized mishti dahi and the effect of storage on these parameters
| Parameters | Mishti dahi (honey 10%)+ Lignan (3.75%) | Control Sample | ||||
| Storage days | SPC Count (log cfu/g) | Coliform Count (log cfu/g) | Yeast and Mould Count (log cfu/g) | SPC Count (log cfu/g) | Coliform Count (log cfu/ml) | Yeast and Mould Count (log cfu/ml) |
| 0th day | 5.11 ±0.06 | 0 | 1.36 ± 0.2 | 5.19 ± 0.05 | 0 | 1.45 ± 0.3 |
| 5th day | 5.24 ±0.04 | 0 | 2.21 ± 0.3 | 5.31 ± 0.01 | 0 | 2.2 ± 0.2 |
| 10th day | 5.38 ±0.03 | 0 | 2.64 ± 0.4 | 5.47 ± 0.01 | 1 ± 0.6 | 2.84 ± 0.2 |
| 15th day | 5.47 ±0.04 | 2 ± 0.8 | 2.87 ± 0.4 | 5.76 ± 0.02 | 2 ± 0.5 | Spoilage found |
| 20th day | 5.69 ±0.05 | 3 ± 0.2 | Spoilage found | 5.98 ± 0.03 | 3 ± 0.1 | Spoilage found |
In Mishti Dahi supplemented with lignan and control sample, there were no presence of coliform till 5th day of storage. This work is in similarity with [9] where they reported that there was no evidence of coliform in samples of pineapple and ginger yogurt. The main factors for coliforms in curd production are microbiological quality of ingredients getting used for curd making after heat treatment of the base milk, i.e., flavorings and starter culture’ cleanliness of the working environment, the hygiene of the operator and equipments used. Similar results were reported by [10], who found no growth of coliform bacteria in dahi samples throughout the storage time. Here in the lignan incorporated mishti dahi showed no presence of coliform even on 10th day of storage whereas less than 3 log cfu/ g coliform seen in the control sample. This can be attributed to the antimicrobial, antibacterial and antifungal property of the lignan and honey as well. It can be seen from table 3 that mishti dahi with lignan had nil count for coliform and remained so even after 10th day storage. According to the IS Standard, a maximum count of 10 log cfu/g of coliform is allowed in mishti dahi.
The Mishti dahi with and without lignan showed significant increases in yeast and mould count. Yeast and mould count of Mishti dahi with Lignan ranged from 1.36 ± 0.2 to 2.87 ± 0.4 log cfu/g. At the beginning mishti dahi containing lignan and control had the lowest yeast and mould count, but after 10 days of storage, yeast and mould growth accelerated in control as compared to sample during next 15 days and hence spoilage was seen earliest in the control sample. From the table 3, it can be concluded that the control sample was spoiled by the 15th day storage whereas developed mishti dahi with lignan was spoiled after 15th day of storage. Hence, lignan incorporated mishti dahi has longer shelf life as compared to the control sample.
Changes in sensorial characteristic of mishti dahi
Changes in the sensorial properties of lignan incorporated mishti dahi sample stored at 4 ͦ C for 15 days are presented in Table 4. The appearance score decreased during storage period shown in table 4. The taste score decreased during storage from 7.6 ± 0.1 to 7.5 ± 0.4. The body and texture score were ranged 7.9 ± 0.2 to 7.7 ± 0.2. Overall, with prolonged storage of mishti dahi the body and texture scores decreased. Colour and appearance scores of the mishti dahi at the 15th day was slightly lower compared to the product at 1st day as there will be a little whey loss and probably due to this there will be a slight change to the surface and ultimately it will scatter the light in a different way. This change may not even significant. Also the score of the colour and appearance that got reduced was within acceptable level.
It can be seen from the table 4 that body and texture of the developed product and control sample both slightly decreased throughout the 15 day’s storage. Overall, with prolonged fermentation the texture scores decreased. The current result was an agreement with the findings of [11]. Also in contrast, [12] found that prolonged storage led to an increase in the body and texture score.
Table 4: Effect of Storage on the Sensory characteristic of optimized mishti dahi
| Parameters | Optimized Mishti Dahi | Control Mishti Dahi | |||||||
| 0th day | 5th day | 10th day | 15th day | 0th day | 5th day | 10th day | 15th day | ||
| Colour and Appearance | 7.6 ± 0.1 | 7.6 ± 0.2 | 7.5 ± 0.1 | 7.5 ± 0.4 | 7.6 ± 0.1 | 7.6 ± 0.2 | 7.5 ± 0.1 | 7.1 ± 0.3 | |
| Taste | 7.6 ± 0.1 | 7.5 ± 0.1 | 7.5± 0.3 | 7.3 ± 0.1 | 7.6 ± 0.1 | 7.5 ± 0.1 | 7.3 ± 0.2 | 6.9 ± 0.2 | |
| Body and Texture | 7.9 ± 0.2 | 7.9 ± 0.1 | 7.7 ± 0.1 | 7.7 ± 0.2 | 7.9 ± 0.2 | 7.9 ± 0.1 | 7.4 ± 0.1 | 7.0± 0.1 | |
| Overall acceptibility | 7.76 ±0.1 | 7.66 ± 0.1 | 7.53 ± 0.2 | 7.5 ± 0.1 | 7.7 ± 0.2 | 7.66 ± 0.1 | 7.4 ± 0.1 | 7.0 ± 0.1 | |
| Parameters | Developed Mishti Dahi | Control Mishti Dahi | ||||||
| Storage days | L* | a* | b* | dE* | L* | a* | b* | dE* |
| 0th day | 85.47 ± 0.05 | -0.49 | 13.57 ± 0.04 | 14.9 ± 0.08 | 86.38 ± 0.03 | -0.8 | 11.37 ± 0.01 | 12.41 ± 0.04 |
| 5th day | 85.44 ± 0.04 | -0.49 | 13.56 ± 0.01 | 14.7 ± 0.07 | 86.34 ± 0.05 | -0.8 | 11.35 ± 0.02 | 12.37 ± 0.04 |
| 10th day | 85.41 ± 0.05 | -0.5 | 13.56 ± 0.06 | 14.7 ± 0.06 | 86.29 ± 0.05 | -0.82 | 11.35 ± 0.02 | 12.37 ± 0.05 |
| 15th day | 85.32 ± 0.02 | -0.51 | 13.53 ± 0.04 | 14.4 ± 0.06 | 86.22 ± 0.04 | -0.84 | 11.32 ± 0.03 | 12.34 ± 0.04 |
Changes in optical properties (color) of mishti dahi
Changes in the colour of lignan incorporated mishti dahi sample stored at 4 ͦ C for 15 days are presented in Table 5. The L*-values for all the samples were not significantly affected by elevating the storage period from 0 to 15 days. The a*- and b*-values of the control sample significantly decreased from −0.8 to −0.84 and 11.37± 0.01 to 11.32 ± 0.03, respectively, when the storage period was increased from 0 to 15 days.
Table 5: Effect of Storage on Colour Parameters of Mishti Dahi
| Parameters | Developed Mishti Dahi | Control Mishti Dahi | ||||||
| Storage days | L* | a* | b* | dE* | L* | a* | b* | dE* |
| 0th day | 85.47 ± 0.05 | -0.49 | 13.57 ± 0.04 | 14.9 ± 0.08 | 86.38 ± 0.03 | -0.8 | 11.37 ± 0.01 | 12.41 ± 0.04 |
| 5th day | 85.44 ± 0.04 | -0.49 | 13.56 ± 0.01 | 14.7 ± 0.07 | 86.34 ± 0.05 | -0.8 | 11.35 ± 0.02 | 12.37 ± 0.04 |
| 10th day | 85.41 ± 0.05 | -0.5 | 13.56 ± 0.06 | 14.7 ± 0.06 | 86.29 ± 0.05 | -0.82 | 11.35 ± 0.02 | 12.37 ± 0.05 |
| 15th day | 85.32 ± 0.02 | -0.51 | 13.53 ± 0.04 | 14.4 ± 0.06 | 86.22 ± 0.04 | -0.84 | 11.32 ± 0.03 | 12.34 ± 0.04 |
Antidiabetic activity of Mishti dahi in honey with and without lignan
Comparison of the α amylase percentage inhibition of the lignan enriched mishti dahi over control revealed that at the concentration of 100µg/ml exhibited 48.41% compared to control which is 17.54%, respectively as per shown in fig 2.
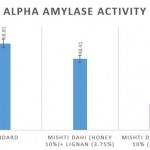 |
Figure 2: Comparison of antidiabetic activity of mishti dahi with honey and lignan incorporated mishti dahi in honey. The results are represented as Mean±S.E. |
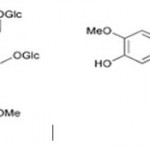 |
Figure 3 |
Conclusion
The present study was designed to develop a neutraceutical flax lignan-supplemented mishti dahi, and to evaluate the effects of adding flax lignan on the physiochemical, microbiological and sensorial properties of the final products during storage. Data from the pH, titratable acidity, moisture content, total solids, whey syneresis, colour, microbiological and sensory analyses obtained from the present study indicated that a 3.75% flax lignan incorporated mishti dahi is having a proper shelf life of 15 days. Also, there were a few evident changes in some of the parameters after 15th day but still it was safe for consumption. To conclude after 20 days’ period of storage, developed mishti dahi gradually started detoriating and marked the end of shelf life. Furthermore, it was found that flax lignan supplemented mishti dahi could also serve as an antidiabetic agent for enhancing human health and treating diabetes. The production of mishti dahi containing flax lignan broadened the utilization of lignan from various sources, and provided a new nutraceutical food that can promote human health.
Acknowledgement
This study was carried out within the Project period of third semester 2015 in SRM University. Author wants to thank all the staffs of the Food Process Department, School of Bioengineering, SRM University, Kattankulathur, Chennai, Tamilnadu, India for their immense support and help throughout the project period.
References
- Sicilia, T., Niemeyer, H.B., Honig, D.M., Metzler, M. Identification and stereochemical characterization of lignans in flaxseed and pumpkin seeds. Journal of Agricultural and Food Chemistry, 2003; 51: 1181–1188.
- Charlet, S., Bensaddek, L., Raynaud, S., Gillet, F., Mesnard, F., Fliniaux, M.A. An HPLC procedure for the quantification of anhydrosecoisolariciresinol, Application to the evaluation of flax lignan content. Plant Physiology and Biochemistry, 2002; 40: 225–229.
- Berhanu, Tsehayneh, A. G. Fermented Ethiopian dairy products and their common useful microorganisms: A review. World Journal of Agricultural Sciences, 2014; 10(3): 121-133.
- Amerine, M.A., Pangborn, R.M., and Roessler, E.B. Principles of sensory evaluation of food.Academic Press. Elsevier, London; UK; 1965; 602 p; ISBN 978-0120561506.
- Amatayakul, T., Frank, S., Nagendra, P, S. Syneresis in set yogurt as affected by EPS starter cultures and levels of solids. International Journal of Dairy Technology, 2006; Volume 59; Issue 3; pages 216–221
- O’Neil, Kleyn, J.M., D.H., and Hare, L.B. Consistency and compositional characteristics of commercial yoghurts. Journal of Dairy Science, 1979; 62: 1032-1036.
- Tarakci, Z., and Kucukoner, E. Physico-chemical, sensory and microbial quality of stirred yogurt prepared with different fruit flavorings. Journal of Food Science and Technology, 2004; 41: 177-181.
- Ostlie, H.M., Helland, M.H., and Narvhus, J.A. Growth and metabolism of selected strains of probiotic bacteria in milk. International Journal of Food Microbiology, 2003;87: 17-27.
- Ihemeje A., Nwachukwu C.N., and Ekwe C. C. Production and quality evaluation of flavoured yoghurts using carrot, pineapple, and spiced yoghurts using ginger and pepper fruit. African Journal of Food Science, 2015; 9(3): 163-169.
- Obi, T.E., Henshaw, F.O., & Atanda, O.O. Quality evaluation of plain-stirred probiotic yoghurt produced from skim and whole milk powder during refrigerated storage, Electronic Journal of Environmental, Agricultural and Food Chemistry, 2010; 9: 1203- 1213.
- Farooq, H., and Haque, Z.U. Effect of sugar esters on the textural properties of nonfat low calorie yoghurt. Journal of Dairy Science, 1992; 75: 2676-2680.
- Keating, K.R., and White, C.H. Effect of alternative sweeteners in plain and fruit-flavored yogurts. Journal of Dairy Science, 1990; 73: 54-62.

This work is licensed under a Creative Commons Attribution 4.0 International License.





