Manuscript accepted on :
Published online on: 12-01-2016
Abbas Zarei and Amin Ebadi*
Department of Chemistry, Kazerun Branch, Islamic Azad University, Kazerun, Iran
DOI : http://dx.doi.org/10.13005/bbra/1919
ABSTRACT: Copper oxide supported on γ-alumina nanoparticles were prepared and were well characterized. In this study catalytic activities of the supported copper oxide for oxidation of cyclohexene to 2-cyclohexene-1-ol, 2-cyclohexene-1-one and cyclohexene oxide with TBHP and H2O2 as the oxidant, in the liquid phase were considered. For the CuO/γ-alumina nanocomposites, acetonitrile as the solvent was employed. The products of the catalysis are 2-cyclohexene-1-ol, 2-cyclohexene-1-one and cyclohexene oxide. The conversion percent of cyclohexene depended on oxidant, solvent and the catalyst. TBHP was found to be better oxidant than H2O2 since minimal destruction of the catalyst and higher conversion of cyclohexene were observed when this oxidant was employed. Under these reaction conditions, the order of catalytic activities is as follows: 10%CuO/γ-alumina > 15%CuO/γ-alumina > 5%CuO/γ-alumina. When the reaction was catalyzed with γ-alumina without any CuO, the reaction yield and conversion were reduced considerably compared to those of supported γ-alumina. With 10% CuO supported on γ-alumina nanoparticles and under our experimental conditions, the conversion percent of cyclohexene is 84.9%.
KEYWORDS: Oxidation; γ-Alumina Nanoparticles; Supported; CuO; Cyclohexene
Download this article as:| Copy the following to cite this article: Zarei A, Ebadi A. Property and Performance of CuO/γ-Alumina Nanocomposites for the Oxidation of Volatile Organic Compounds. Biosci Biotechnol Res Asia 2015;12(3) |
| Copy the following to cite this URL: Zarei A, Ebadi A. Property and Performance of CuO/γ-Alumina Nanocomposites for the Oxidation of Volatile Organic Compounds. Biosci Biotechnol Res Asia 2015;12(3). Available from: https://www.biotech-asia.org/?p=3673 |
Introduction
Volatile Organic Compounds (VOCs) emitted in air during industrial processes using solvents, polymers and resins such as those involved in painting and coating operations are considered as considerable atmospheric pollutants generating damaging effects1,2. According to one of the most widely accepted definitions, a wide variety of organic compounds, such as aliphatic, aromatic and chlorinated hydrocarbons, aldehydes, ketones, esters, organic acids and alcohols can be considered as VOC. Volatile organic compounds (VOCs) are hazardous for the environment, because of their high volatility and insolubility in air, and their ability to travel long distance from their emission points and to be transformed in the atmosphere in other compounds even more hazardous for humans, fauna and flora. For instance, many VOC participate in the formation of photochemical smog, in the greenhouse effect or have been found to be an important cancer risk factor, and to be implicated in neurological problems in urban children3-5.
The catalytic oxidation of volatile organic compounds (VOC) present in low concentrations in effluent gases is a main technique to control VOC emissions6. During the last decade, there have been significant advances in the oxidation of volatile organic compounds by oxidant7-10. However, efficient and selective methods for the catalytic functionalization of volatile hydrocarbons still remain a main challenge in both synthetic and industrial chemistry. The allylic oxidation of olefin into α,β-unsaturated ketones is a very important reaction as the fundamental technology in the chemical industry worldwide11. In particular, the oxidation products of cyclohexene and their derivatives are important in organic synthesis due to the presence of a highly reactive carbonyl group, which is used in cycloaddition reactions12-14. Great efforts have been devoted to the oxidation of cyclohexene in the past years15-19.
Transition metal complexes are well known for their catalytic activities in the oxidation as well as reduction of organic hydrocarbons and for the synthesis of suitable chemicals. Their immobilizations on solid supports provide certain additional characteristic properties such as selectivity, thermal stability and easy separation from the reaction mixture and recycle ability. The immobilized materials are important to develop new environment friendly technologies20-23. In these catalysts, Al2O3, TiO2, SiO2 and ZrO2 are commonly utilized as the supports. Bulk oxides in general cannot be used in industrial processes as they impart poor thermal stability that lead to fast deactivation of the catalyst. Furthermore, it is also known that bulk CuO leads to high combustion of organic molecules to carbon oxides24. The oxidation of cyclohexene in the presence of NiO/MCM-41 nanocomposites resulted in the formation of 2-cyclohexene-1-ol and 2-cyclohexene-1-one25. It has been reported that Au nanocatalyst on modified bentonite and silica the effective catalysts for solvent free oxidation of cyclohexene with molecular oxygen26. In another report Mahmoodi Hashemi and coworkers have employed novel silica-functionalized ammonium tungstate as catalysts for cyclohexene oxidation27.
However, to the best of our knowledge, there is no report for application of CuO supported on nano-sized γ-alumina for partial oxidation of cyclohexene with H2O2 and TBHP. In this work CuO supported on nano-sized γ-alumina were reported as catalysts for aerobic oxidation of cyclohexene to 2-cyclohexene-1-ol, 2-cyclohexene-1-one and cyclohexene oxide in the liquid phase.
Experimental
Instrument and Reagents
Powder X-ray diffractions were performed by a Siemens D 5000. Specific surface area was measured by BET techniques in liquid N2 temperature by a Strohlien. The surface morphology of the samples was obtained using a Jeol-JSM-5610 LV scanning electron microscopes (SEM). The reaction products of oxidation were identified by GC-MS (Finnigan TSQ-7000) and were analyzed by GC (Shimadzu 8A). All the reagents were commercial grade obtained from Merck. None of the oxidation products were found in the cyclohexene before the oxidation reaction.
Preparation of the Catalysts
Aluminum nitrate {Al(NO3)3.9H2O}, aqueous ammonia {NH3.H2O} and deionized water were used as starting chemicals. Two hundred milliliters of deionized water was taken in a 2 l capacity round-bottom flask and stirred well using magnetic stirrer. Then, aluminum nitrate (1.8 M) solution and (11.5 M) solution of aqueous ammonia were added to 200 ml of deionized water drop by drop to precipitate Al cations in the form of hydroxides. The temperature was maintained ~60 °C during precipitation/digestion experiment. The pH after precipitation was found to be in the range of 5.5-6.5. The precipitates were further digested at 60 °C for 1 h. After the alumina gel was formed, it was filtered and washed by distilled water. Then, (0.1 M) copper nitrate Cu(NO3)2 aqueous solution was added to the alumina-gel. This gel was stirred and homogenized and was placed in an oven under temperature of 110 °C for 24 h. The mixture was then heated 2 °C/min till the temperature reached 550 °C and the mixture was kept at this temperature for 6 h.
Experimental Procedure
In a typical procedure, a mixture of 0.5 g catalyst, 25 ml solvent of acetonitrile and 10 mmol cyclohexene was stirred under nitrogen in a 50 ml round bottom flask equipped with a condenser and a dropping funnel at room temperature for 30 min. Then 16 mmol of TBHP (solution 80% in di-tert-butylperoxide) or H2O2 (30% in H2O) was added as oxidizing reagents. The resulting mixture was then refluxed for 8 h under N2 atmosphere. After filtration, the solid was washed with solvent and then the reaction mixture was analyzed by GC. Products identification was done with GC-MS and confirmed by comparison of their retention times with authentic commercial samples of these compounds.
Results and Discussion
Characterization of the Catalysts
The XRD pattern presented in Figure 1 indicates that γ-alumina nanoparticles are formed. There is no significant change in the XRD pattern with 10 wt.% CuO supported on γ-alumina nanoparticles which confirms that CuO dispersed through pores does not change the γ-alumina structure.
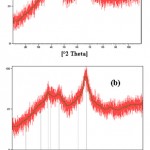 |
Figure 1: XRD patterns of (a) γ-alumina nanoparticles, and (b) 10 wt.% CuO/nano-γ-alumina. |
Scanning electron micrograph (SEM) of a typical sample of 10% CuO/nano-γ-alumina is shown in Figure 2. It is clarified from Figure 2 that the sizes of the particles are in the ranges of 35-50 nm. This result was coincident with the particle sizes calculated from the Scherrer equation. Similar images were obtained for the other catalysts. Specific surface area measured with BET method was 198 m2/g for γ-alumina and 175 m2/g for 10% CuO/nano-γ-alumina. This reduction in specific surface area for the supported CuO may be an indication of encapsulation of CuO in the nano-γ-alumina pores.
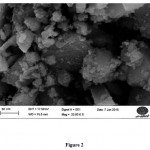 |
Figure 2: SEM photograph of 10% CuO/nano-γ-alumina. |
Catalytic Oxidation of Cyclohexene
The use of TBHP as an oxidant was based on the earlier studies on the oxidation of hydrocarbon28; this oxidant was found to cause minimal destruction of the catalyst, and to give better selectivity of the products. For comparative purposes, H2O2 was also employed as an oxidant. The solvent of acetonitrile was employed for the catalysis, since all the reagents dissolved and gave the highest yields of the products. The performance of the set of samples prepared as heterogeneous catalysts for the oxidation of cyclohexene was tested using hydrogen peroxide (H2O2) and tert-butylhydroperoxide (TBHP) as oxidizing reagents (Table 1). In all the cases the only products observed were 2-cyclohexene-1-ol, 2-cyclohexene-1-one and cyclohexene oxide. In the presence of 10% CuO/nano-γ-alumina, conversion percentage of cyclohexene was 84.9% with TBHP as an oxidant. Contrastive experiment results show that cyclohexene oxidation with TBHP and H2O2 did not occur in the absence of the catalyst under the same reaction condition and unsupported γ-alumina has shown lower catalytic activity than the supported catalyst.
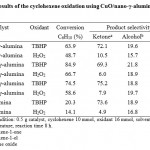 |
Table 1: Results of the cyclohexene oxidation using CuO/nano-γ-alumina as catalysts. |
Influences of Reaction Time on Cyclohexene Oxidation Reaction
In this experiment, the change in conversion percentage of cyclohexene in the presence of TBHP oxidant and 10% CuO/nano-γ-alumina catalyst was monitored and plotted with respect to time (Figure 3). The reaction was carried out at reflux temperature for 8 h with 0.5 g catalyst and 10 mmol cyclohexene and 16 mmol TBHP in a round bottom flask and some samples was drawn out at regular intervals and analyzed by GC. Figure 3 shows that the conversion of cyclohexene increases continuously until 84.7% as time increases and then remains constant after 7 h, therefore duration about 7-8 h is proper reaction time.
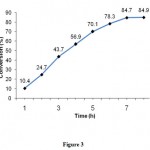 |
Figure 3: The effect of reaction time on cyclohexene conversion. |
Influences of the Loading Amount of Copper Oxide on Cyclohexene Oxidation Reaction
For investigation of the loading effect CuO on the conversion and selectivity of the products three catalysts were tested. In Table 1, details of the conversion and selectivity of the products for each catalyst are shown. It is observed that maximum conversion and selectivity for 2-cyclohexene-1-ol and 2-cyclohexene-1-one occurs with the catalyst of 10 wt.% CuO. It is known that copper oxide can be highly dispersed on γ-alumina at 10 wt.% loading. A drop of conversion of cyclohexene and selectivity for 2-cyclohexene-1-ol and 2-cyclohexene-1-one of the catalyst with higher loadings than 10 wt.% is possibly due to a more reduction of the specific surface area of the catalyst. Under these reaction conditions, the order of catalytic activities is as follows:
10 wt.% CuO / nano-γ-alumina > 15 wt.% CuO / nano-γ-alumina > 5 wt.% CuO / nano-γ-alumina.
Influences of Oxidant Type on Cyclohexene Oxidation Reaction
Figure 4 shows that the reactivity of the cyclohexene toward oxidation with TBHP and H2O2 on CuO supported on nano-γ-alumina catalysts depend on type of oxidant. tert-Butylhydroperoxide (TBHP) was found to be a more convenient oxidizing reagent due to weaker O_O bond than hydrogen peroxide (H2O2). Oxidation of cyclohexene with TBHP gave 2-cyclohexene-1-one as the main product. The formation of the allylic oxidation products 2-cyclohexene-1-one and 2-cyclohexene-1-ol shows the preferential attack of the activated C_H bond over the C=C bond. TBHP as oxidant promotes the allylic oxidation pathway and epoxidation is minimized.
When the oxidant was changed to hydrogen peroxide, the oxidation occurred on the double bond and cyclohexene oxide obtained as the main product. Although both TBHP and H2O2 oxidize cyclohexene in the presence of CuO supported on nano-γ-alumina, but more H2O2 and not TBHP gave epoxidation of cyclohexene under the similar conditions leads us to conclude that the two types of reactions do not occur via a common intermediate. As Valentine and his co-workers have pointed out one possible explanation is that the species responsible for the cyclohexene oxidation are the products formed from cleavage of the O_O bond, whereas, the epoxidation reaction occurs by a direct reaction of olefin with coordinated HOO radical. Since the O_O bond of HOOH is 5 kcal/mol stronger than TBHP, an HOO complex is expected to have a higher activation energy for O_O bond cleavage than a TBOO complex and therefore, to have a longer lifetime29.
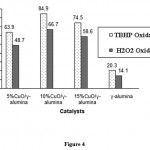 |
Figure 4: The effect of oxidant type on cyclohexene conversion in the presence of acetonitrile as the solvent. |
Conclusion
The nano-γ-alumina encapsulated copper oxides, CuO/nano-γ-alumina, have been synthesized and characterized. Catalytic activities of these nanocomposites have been tested for the partial oxidation of cyclohexene to 2-cyclohexene-1-ol and 2-cyclohexene-1-one using tert-butylhydroperoxide (TBHP) as the oxidant, in the presence of acetonitrile solvent. A maximum of 84.9% conversion of cyclohexene has been achieved with 10 wt.% CuO/nano-γ-alumina using TBHP as the oxidant, where selectivity of 2-cyclohexene-1-one and 2-cyclohexene-1-ol are 69.3% and 21.8%, respectively. They are stable, reusable and can be easily separated after the reaction, which endow copper oxides supported on nano-γ-alumina with a bright future in industrial applications.
Acknowledgement
We gratefully acknowledge financial support from the Research Council of kazerun Branch, Islamic Azad University.
References
- De Nevers,, Air Pollution Control Engineering, McGraw-Hill, 1995.
- Kosusko, M., Numez, C.M., Destruction of volatile organic compounds using catalytic oxidation, Air Waste Manage. Assoc., 1990; 40(2): 254-9.
- Schneider, S.H., Global Warming, Vintage Books, New York: 1989.
- Tancrede, M., Wilson, R., Zeise, L., Crouch, E.A.C., The carcinogenic risk of some organic vapors indoors: A theoretical survey, Environ., 1987; 21(10): 2187-2205.
- USEPA Inventory of US Greenhause Gas Emissions and Sinks: 1990-1997, US EPA, Washington, DC, 1999.
- Horsley, J.A., Catalysts for the Elimination of Volatile Organic Compounds, Nonhalogenated Compounds, Catalytica Environmental Report Number E4, Catalytica Studies Divison, 1993.
- Hill, C.L., Activation and Functionalization of Alkanes, Academic Press, New York: 1989.
- Sheldon, R.A., Kochi, J.K., Metal-Catalyzed Oxidation of Organic Compounds, Academic Press, New York: 1981.
- Simandi, L.L., Catalytic Activation of Dioxygen by Metal Complexes, Kluwer Academic, Dordrecht: 1992.
- Barton, D.H.R., Martell, A.E., Sawyer, D.T., (eds): The Activation of Dioxygen and Homogeneous Catalytic Oxidation, Plenum, New York: 1993.
- Cainelli, G., Cardillo, G., Chromium Oxidation in Organic Chemistry, Springer verlag, Berlin: 1984.
- Wiberg,B., Oxidation in Organic Chemistry, Academic press, New York: 1965.
- Waters, W.A., Mechanisms of Oxidation of Organic Compounds, Methuen, London: 1964.
- Smith, A.B., Konopelski, J.P., Total synthesis of (+)-quadrone: assignment of absolute stereochemistry, Org. Chem., 1984; 49(21): 4094-5.
- Lau,-H., Caps, V., Yeung, K.-W., Wong, K.-Y., Tsang, S.C., A novel MCM-41-supported manganese(III) complex with nitrogen donor ligand for cyclohexene oxidation, Micropor. Mesopor. Mater., 1999; 32(3): 279-285.
- Wu, P., Tatsumi, T., Komatsu, T., Yashima, T., A novel titanosilicate with MWW structure: II. catalytic properties in the selective oxidation of alkenes, Catal., 2001; 202(2): 245-255.
- Sakthivel,, Dapurkar, S.E., Selvam, P., Allylic oxidation of cyclohexene over chromium containing mesoporous molecular sieves, Appl. Catal. A: Gen., 2003; 246(2): 283-293.
- Sehlotho,, Nyokong, T., Catalytic activity of iron and cobalt phthalocyanine complexes towards the oxidation of cyclohexene using tert-butylhydroperoxide and chloroperoxybenzoic acid, J. Mol. Catal. A: Chem., 2004; 209(1-2): 51-57.
- Liu, Y., Murata, K., Inaba, M., Nakajima, H., Koya, M., Tomokuni, K., Catalytic oxidation of cyclohexene by molecular oxygen over isopolyoxometalates, Lett., 2004; 33(2): 200-201.
- Sherington, D.C., Hodnett, B.K., Keybett, A.P., Clark, J.H., Smith, K., (Eds.): Supported Reagents and Catalyst in Chemistry, Royal Society of Chemistry, Cambridge: 1998; pp. 220-8.
- Soai, K., Watanable, M., Yamamoto, A., Enantioselective addition of dialkylzincs to aldehydes using heterogeneous chiral catalysts immobilized on alumina and silica gel, Org. Chem., 1990; 55(16): 4832-5.
- Joseph, T., Deshpande, S.S., Halligudi, S.B., Vinu, A., Ernst, S., Hartmann, M., Hydrogenation of olefins over hydrido chlorocarbonyl tris-(triphenylphosphine) ruthenium(II) complex immobilized on functionalized MCM-41 and SBA-15, Mol. Catal. A: Chem., 2003; 206(1-2): 13-21.
- Joseph, T., Halligudi, S.B., Oxyfunctionalization of limonene using vanadium complex anchored on functionalized SBA-15, Mol. Catal. A: Chem., 2005; 229(1-2):241-7.
- Routray, K., Reddy, K.R.S.K., Deo, G., Oxidative dehydrogenation of propane on V2O5/Al2O3 and V2O5/TiO2 catalysts: understanding the effect of support by parameter estimation, Catal. A: Gen., 2004; 265(1): 103-113.
- Ebadi, A., Mozaffari, M., Shojaei, S., Aerobic oxidation of cyclohexene catalyzed by NiO/MCM-41 nanocomposites in the gas phase, Chem. Sci., 2014; 126(4): 989-996.
- Shahabi nejad, M., Ghasemi, , Martínez-Huerta, M.V., Ghiaci, M., Synthesis and characterization of Au nanocatalyst on modifed bentonite and silica and their applications for solvent free oxidation of cyclohexene with molecular oxygen, J. Mol. Catal. A: Chem., 2015; 406:118-126.
- Vafaeezadeh, M., Mahmoodi Hashemi, M., Simple and green oxidation of cyclohexene to adipic acid with an efficient and durable silica-functionalized ammonium tungstate catalyst, Comm., 2014; 43: 169-172.
- Grootboom, N., Nyokong, T., Iron perchlorophthalocyanine and tetrasulfophthalocyanine catalyzed oxidation of cyclohexane using hydrogen peroxide, chloroperoxybenzoic acid and tert-butylhydroperoxide as oxidants, Mol. Catal. A: Chem., 2002; 179(1-2): 113-123.
- Nam, W., Ho, R., Valentine, J.S., Iron-cyclam complexes as catalysts for the epoxidation of olefins by 30% aqueous hydrogen peroxide in acetonitrile and methanol, J. Am. Chem. Soc., 1991; 113(18): 7052-4.

This work is licensed under a Creative Commons Attribution 4.0 International License.





