Manuscript accepted on :
Published online on: 21-12-2015
Fingolimod (FTY720) As an Anti-Multiple Sclerosis Oral Ultimate Therapy
Sara T. Alrashood1*, Fadilah S. Aleanizy2, Fulwah Alqahtani2, Ahad Abushal3, Noor AlSalama3, Ghada S. Hassan4
1Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, P.O.Box 2457, Riyadh 11451, Saudi Arabia. 2Department of Pharmaceutics, College of Pharmacy, King Saud University, P.O.Box 2457, Riyadh 11451, Saudi Arabia. 3College of Pharmacy, King Saud University, P.O.Box 2457, Riyadh 11451, Saudi Arabia. 4Department of Medicinal Chemistry, Faculty of Pharmacy, Mansoura University, P.O.Box 35516, Mansoura, Egypt.
ABSTRACT: Multiple Sclerosis (MS) is a chronic progressive autoimmune disease. It has complex symptoms and challenges. Patients suffer the most from the inconvenience and intolerability till the old injectable disease modifying therapies. Therefore our research is highlighting the scoop on the newly available treatment using the oral therapies proceeding from their chemical nature and structure to prove their superiority to the traditional ones in controlling MS progression. This can lead to an improvement inlife quality for MS patients with the enhanced tolerability for oral drugs that is best achieved by using Fingolimod that is considered as the first approved oral drug for this disease.
KEYWORDS: Multiple Sclerosis (MS); Autoimmune disease; Fingolimod; Therapy
Download this article as:| Copy the following to cite this article: Alrashood S. T, Aleanizy F. S, Alqahtani F, Abushal A, AlSalama N, Hassan G. S. Fingolimod (FTY720) As an Anti-Multiple Sclerosis Oral Ultimate Therapy. Biosci Biotech Res Asia 2015;12(2) |
Introduction
Multiple sclerosis (MS) is an inflammatory disease that affects and cause damage to the nerve cells covers in the brain and spinal cord. This damage results in the disability of communication in the nervous system, leading to a wide range of signs and symptoms1 for examples; physical, mental2 and sometimes psychiatric problems3. In another word, it is an idiopathic putatively autoimmune, chronic inflammatory degenerative disease having demyelinating effect on of central nervous system (CNS) with genetic, infectious, immunologic and environmental effects. The immune system attacks the nerve cells of the CNS and that affect both white and gray matters4. It has different forms that occur with new symptoms either occurring in the form of isolated attacks (relapsing forms) or increasingby time in a progressive way. Symptoms may disappear completely between attacks; in some cases, permanent problems often occur, especially as the disease advances5.
The MS can appear between 20 and 40 years, this early appearance makes it the second most common cause of disability in young adults. The female/male ratio in this group approaches 3:1 and may be increasing. MS causes disabling physical symptoms involving mobility and coordination problems, vision problems, cognitive dysfunction, fatigue, numbness, and pain. Quality of life may be further reduced by mood disorders and limitations in social functioning6.Considering the line of treatment, multiple sclerosis is untreatable, only several attemptsfor function improvement after an attack with prevention of further attacks.
On the other hands, not only these drugs have moderate effect but also they can have adverse effects in addition to the poor toleration produced. Prediction of the long-term use is very difficult, good outcomes appear mostly in women, and especially who has early development of the disease early in life, in addition to those with a relapsing course, and finally those who experienced low number of attacks. Some expectations were discussed about the life-time of the patients over the unaffected ones, it was found that it is on average 5 to 10 years lower than healthy population7. As mentioned before, there is no acceptable cure for this disease, only several therapies have proven to help improvement of the life style in tolerance the possible attacks. The primary aims of the used drugs are to return the normal function after a possible attack, in addition to the attempt to preventfurther attacks, and finally trying to prevent the irreversible disability.
The usual therapy used during symptomatic attacks is the administration of high doses corticosteroids by intravenous route, such as methylprednisolone. Similarly, treatment with oral corticosteroids gives the same effect and safety profile8. On the other hand,corticosteroid treatments appear to be ineffective in the long term recovery contrast for the short term therapy.Furthermore, treatment of attacks that do not respond to corticosteroids relies on the use of plasmapheresis1. Other lines of treatments including Disease Modifying Therapies (DMTs) either for Relapsing remitting multiple sclerosis or Progressive multiple sclerosis. In case of DMTstheir efficacy is determined by the reduction of relapses rate and decreased accumulation of brain lesions on Magnetic Resonance Imaging (MRI)9.The First line of treatment includes Interferon a-IFNb-1b (betaferon,Extavia), or b-IFNb-1a (Avonex, Rebif), and Glatiramer acetate (GA) while the Second line involves the use of Natalizumab (Tysabri), Fingolimod (Gilenya), Triflunamide (Aubagio), and Dimethyle Fumarate (Tecfidera)10.
In September 2010, Fingolimod was approved as oral DMT for relapsing multiple sclerosis,. It is a sphingosine-1-phosphate receptor (S1P) agonist a G-protein coupled receptor- binding to 4 of the 5 S1P receptor subtypes and acting as a functional antagonist. The drug is highly lipophilic but well absorbed orally regardless food and fatty meals (93% oral bioavailability)10. In vivo, it’s rapidly and reversibly phosphorylated by sphingosine kinase-2 to its active metabolite Fingolimod phosphate. Fingolimod is mtaabolized by (CYP4F2) giving low potential for drug-drug interactions as few numbers of drugs are metabolized by this enzyme11.
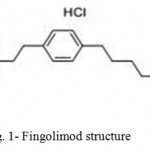 |
Figure 1: Fingolimod structure |
It is used as one 0.5 mg oral capsule once daily.It is indicated as single DMT in highly active relapsing remitting MS; this group of patients includes those who are treated with a beta-interferon but still suffer from high disease activity. These patients do not show any response to a full and adequate course of total one year of treatment using beta-interferon, and they still have had at least 1 relapse in the previous year while on therapy, and have at least 9 T2-hyperintense lesions in cranial MRI or at least 1 Gadolinium-enhancing lesion12. A term “non-responder” is used for patients who remain unchanged or suffer from episodes of increased relapse rate or ongoing severe relapses, as compared to the previous year, in addition to patients who have 2 or more disabling relapses in one year named rapidly evolving severe relapsing remitting multiple sclerosis, and with 1 or more Gadolinium enhancing lesions or a significant increase in T2 lesion load as compared to a previous recent MRI13. The objectives of this research is to conduct a case study in which the Fingolimod was used, collection of data and observation will lead to a conclusion that can be used for the future development and treatment regimen.
Materials and Methods
A case study was conducted, using patients’ data on Fingolimod therapy and compare it with other patients’ data on Interferon (INF) therapy, for 6-12 months period of time, and between age 20-40 years. All patients informed consent was obtained for using their data only, they required their names being blinded. The data was collected from three different sites, King Khalid University Hospital (KKUH),King Faisal Specialty Hospital(KFSH) both in Riyadh, and international MS clinic in Germany. The data collection was based on observing the patients for enhanced tolerability and adherence to regimen with decreased relapses rate, disability progression and improved lesions on MRI. The comparison is made between INFs and Fingolimod aiming for better MS patients’ Quality of life and to prove the superiority of oral DMTs to the injectable traditional ones. A close attention was paid for Fingolimodcardiotoxic side effects, macular edema, and other major side effects as well. Finally, synthesis of such drug involves scheme of a facile six-step beginning with readily available and inexpensive starting material(diethyl acetamidomalonate), that produces the hydrochloride salt of the drug in an acceptable yield. (scheme1)
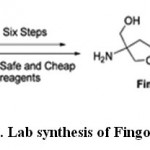 |
Scheme 1: Lab synthesis of Fingolimod HCl |
Results and Discussion
Starting with the patients sample collected for the case study, 43% of the female patients started their therapy using Fingolimod while the rest of females switched from the treatment with interferons (INF) to the test drug. On the other hands, the male patients were classified into 3 groups: those that started with Fingolimod, other group switched from avonex to the test drug, and the final group switched from Rebif to the test drug(Fig.2 and 3).
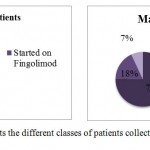 |
Figure 2: Represents the different classes of patients collected for the case study |
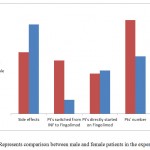 |
Figure 3: Represents comparison between male and female patients in the experiments. |
A comparison was made between male and female patients under investigations, it appears that the percentage that switched from INF to fungolimod in females is much greater than those in male. On the other hands, the side effects of the drugs appeared in such a large percentage in males than females (Fig. 4).
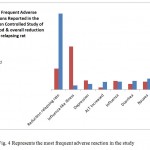 |
Figure 4: Represents the most frequent adverse reaction in the study |
According to the data obtained many of the side effects recorded lower percentage in interferon models than that of fingolimod ones, such as nausea, diarrhea and ALT increased. On the other hands, fingolimod recorded lower percentages in most concerning side effects such as influenza, depression and influenza-like sickness,in addition to very high percentage on the reduction of relapsing rate.
From the study obtained, some items were recommended to be monitored including
Heart Rate
Bradycardia appears after the first dose and lasts for 6 hours. The mean decrease in heart rate was calculated at 6 hours after the first 0.5 mg dose and it was approximately 13 bpm. This disadvantage disappeared by continued dosing within 1 month.
Infections
Complete Blood Cell count (CBC) before starting therapy with Fingolimod. It reduces peripheral lymphocyte counts to 20%-30% of baseline. Treatment should not be initiated in patients with active acute or chronic infections. Coadministration of ketoconazole (an antifungal), a CYP4F2 inhibitor increases the area under the curve (AUC) of Fingolimod and its metabolite Fingolimod phosphate. This requires dosage adjustments (Table-1).
Table 1: Summary of Efficacy Data From Pivotal Controlled Trials for Recently Developed Multiple Sclerosis Disease-Modifying Therapies.
| Characteristic | Fingolimod | Teriflunomide | Dimethyl fumarate |
| Brand name | Gilenya | Aubagio | Tecfidera |
| Year approved | 2010 | 2012 | 2013 |
| Dose | 0.125 or 0.5 mg | 7 or 14 mg | 240 mg |
| Route | Oral | Oral | Oral |
| Frequency | Daily | Daily | BID |
| Relative Risk Reduction | 54% | 31% | 51%-53% |
| Absolute Risk Reduction | 0.18 | 0.37 | 0.17 |
| Numbers needs to treat | 2 | 5 | 6 |
Macular Edema
ophthalmologic evaluation especially with diabetic patients should be performed before starting therapy with fingolimod and at 3 to 4 months after.
Cautions must be considered with patients over 65 years.
Patients with cardiovascular problems such as ischemic heart disease, or heart failure, who have type II second-degree, or third-degree AV block or sick sinus syndrome should not experience such medication, unless the patient has a functioning pacemaker.
No dose adjustment is needed in moderate-severe renal failure, or with hepatic impairment.In case of drug interruption first dose effect can reappear and it requires monitoring when the treatment is reinitiated
Conclusion
Fingolimod is a promising oral agent for use in the treatment of relapsing forms of MS. It showed positive outcomes on phase 3 clinical trials and by that it captured the era on the oral MS therapy and it opened the gate for the dug design and discovery for designing more MS oral DMTs. Therefore, we synthesized FingolimodHCl and studied its chemical nature, and conducted our study using 6-12month patients data that showed a reduction in relapsing rate and MRI lesions, also a decreased risk of disability progression with enhanced overall patient quality of life in comparison with Interferons and other DMTs. Fingolimod therapy requires Careful patient selection and close monitoring that is necessary to avoid toxicities.
Acknowledgements
We thankfully appreciate Professor Ahmed AlRasheed for patients Data from MS clinic in Germany, Also the heads of King Khalid University Hospital (KKUH), and King Faisal Specialty Hospital (KFSH) and information center in Riyadh for the ease of access to patients’ data.
References
- Compston, A., Coles A. Multiple sclerosis. Lancet.,2008;372 (9648): 1502–17.
- Compston, A., Coles, A. Multiple sclerosis. Lancet.,2002;359 (9313): 1221–31.
- Nosarti, C., Reichenberg, A., Murray, R. Preterm birth and psychiatric disorders in young adult life. Arch. Gen. Psychiatry., 2012; 69(6): 610-617
- Noseworthy, J.H., Lucchinetti, C., Rodriguez, M., Weinshenker, B.G., Multiple sclerosis. N. Engl. J. Med., 2000; 343(13):938-952.
- Lublin, FD.,Reingold, SC. Defining the clinical course of multiple sclerosis. Results of an international survey. Neurology 1996; 46: 907–911.
- Sawcer, S., Hellenthal, G., Pirinen, M. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature., 2006; 476(7359):214-219.
- Weinshenker, B.G. Natural history of multiple sclerosis. Annals of Neurology., 1994;36:6–11.
- Burton, J.M., O’Connor, P.W., Hohol, M., Beyene, J. Oral versus intravenous steroids for treatment of relapses in multiple sclerosis. Cochrane Database of Systematic Reviews.,2012;(12): 140-148.
- Orton, S.M., Herrera, B.M., Yee, I.M. Canadian Collaborative Study Group. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol., 2006; 5(11):932-936.
- Miron, V.E., Schubart, A., Antel, J.P. Central nervous system-directed effects of FTY720 (fingolimod). J. Neurol. Sci., 2008; 274(1-2):13-17.
- Brinkmann, V. FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. Br. J. Pharmacol., 2002; 158(5):1173-1182.
- Chun, J., Hartung, H.P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin.Neuro.Pharmacol., 2010; 33(2):91-101.
- Khatri, B., Barkhof, F., Comi, G. 2010. Oral fingolimod (FTY720) reduces the rate of relapses that require steroid intervention or hospitalization compared with intramuscular interferonβ-1a: results from a phase III study (TRANSFORMS) in multiple sclerosis. 62nd American Academy of Neurology Annual Meeting; April 10-17, 2010; Toronto, Canada.

This work is licensed under a Creative Commons Attribution 4.0 International License.





