Manuscript accepted on : 14-Dec-2018
Published online on: 25-12-2018
Plagiarism Check: Yes
Prevalence of Cysticercus Ovis Among Slaughtered Goats in Makkah, Saudi Arabia
Areej O. Bakhraibah and Muslimah N. Alsulami
Department of Biology, Faculty of Science, University of Jeddah, Saudi Arabia.
Corresponding Author E-mail: m-n-solima@hotmail.com
DOI : http://dx.doi.org/10.13005/bbra/2701
ABSTRACT: Cysticercus ovis the intermediate stage of a canine tapeworm, Taenia ovis, produces cystic lesions in the skeletal and cardiac muscle of goats. This study was carried out to determine the prevalence of cysticercus ovis in goats collected from Al Kakee’s Slaughter, Makkah, Saudi Arabia. A number of goats were collected for examination, a total of 51,750 locally raised goats and 61,911 imported goats. The experiment started at May 2017 and ended in April 2018. The results revealed that cysticercus ovis is common among imported goats more than local goats as implied in the tables attached below. As well as the evidence that the highest rate of infections was found during the warm months of summer.
KEYWORDS: Cysticercus Ovis; Goats; Infection; Prevalence; Slaughtered; Veterinary Significance
Download this article as:| Copy the following to cite this article: Bakhraibah A. O, Alsulami M. N. Prevalence of Cysticercus Ovis Among Slaughtered Goats in Makkah, Saudi Arabia. Biosci Biotech Res Asia 2018;15(4). |
| Copy the following to cite this URL: Bakhraibah A. O, Alsulami M. N. Prevalence of Cysticercus Ovis Among Slaughtered Goats in Makkah, Saudi Arabia. Biosci Biotech Res Asia 2018;15(4). Available from: https://www.biotech-asia.org/?p=32112 |
Introduction
Cysticercosis, a parasitic infection caused by the larval form of the pork tapeworm. Cysticercus Ovis, also known as Taenia Ovis, is a parasite from the cestodes family that uses sheep, goats and cattle (livestock) as their intermediate host. Cysticercosis has a complex life cycle. The larval infection, cysticercosis, is transmitted through the fecaloral route. Eggs from the adult tapeworm T. solium, which are directly infectious, are shed in the feces of a human tapeworm carrier and subsequently ingested by pigs, the usual intermediate host (Sorvillo et al .,2007). The oncosphere embryos emerge from the eggs, penetrate the intestinal wall, and are disseminated by the bloodstream to various tissues where the larval stage, or cysticercus, develops. The cycle is completed when humans, the only naturally infected definitive host, consume raw or undercooked pork containing cysticerci, which attach to the small bowel and develop into the adult tapeworm. However, humans may also become infected with the larval stage when eggs are ingested, typically in contaminated food or water. Neurocysticercosis, the most severe form of the disease, occurs when larvae invade tissue of the central nervous system. Cysticercus ovis, the intermediate stage of a canine tapeworm, Taenia ovis, produces cystic lesions in the skeletal and cardiac muscle of sheep which, if numerous, will result in the condemnation of an entire carcass (DeWolf et al., 2012). Cysticercus ovis can be found in dogs (Jenkins et al., 2018) known as Echinococcus and in pigs known as Tenia Solium and can be transferred to sheep and cattle (Garedaghi et al., 2012), even deers (Al-Sabi et al., 2013). When the meat of animals is infected with the C.ovis and consumed by humans it can cause cysticercosis disease or taeniasis when found in the small intestines (Neghab et al., 2006; Neva and brown, 1994) and other organs of the human body. Thus, humans are considered the definitive host of the cysticercus ovis (Eckert & Deplazes, 2004). C. ovis is a thin walled cyst like structure that is filled with fluids, approximately 1 cm in diameter. The cyst in the muscle will degenerate over time and calcify, forming a small noodle known as sheep measles (DeWolf et al., 2012).
The infection usually leads to a lower production rate and in some cases of heavy infection, the death of the animals hosting the parasites (Radfar et al., 2005). Unfortunately, in some cases the condemnation of the entire carcasses is essential when the rate of contamination is high (Hashemnia et al., 2016): (Thompson & Lymbery, 1995).
Preventing and curing the disease is an essential move that needs to be taken as soon as possible because parasites such as the cysticercus ovis can be prevented or cut down by various methods (Anna Erickson, 2018). Although, most countries do not have enough data to implement disease control campaigns (Zheng, 2016). Condemnation of the meat causes a catastrophe in the meat industry especially when the rate of infection is high (Cabaret et al., 2002) It is a major economical loss to the meat industry as well as a public health catastrophe (EARO, 2000).
Materials and Methods
Field Study Area
This cross-sectional study was carried out in one of the biggest abattoir in Makkah province in west of Saudi Arabia, on the east coast of the Red Sea, the gateway to the Two Holy Mosques and the main port in the Kingdom (Fig 1).
The nature of its climate is cool in winter 20°C hot in summer 42°C. The abattoir was visited periodically to examine the slaughtered animals Cysticercus Ovis for the presence of goats.
Animals Number Examination
A number of goats were collected for testing, a total of 51,750 locally raised goats and 61,911 imported goats. All tested goats were from Al Kakee’s slaughter, Makkah, Saudi Arabia. The period of testing began in May 2017 to April 2018, therefore the experiment was conducted for one whole year.
51,750 local goats were slaughtered during the period of experiment from May 2017 to April 2018, along with a total 61,911 imported goats were slaughtered as well during the same period of experiment. Both groups of goat’s meat has gone through a visual inspection with the aid of a manual lens of the mesentery, peritoneal cavity, liver, lungs, kidneys, striated muscles, heart, femoral muscle, diaphragm muscle, tongue and the head of each carcass for the presence of the Cysticercus ovis.
Data Analysis
The data were analyzed using Chi-square analysis to compare the infection rate capacity between infected and non-infected goats. All statistical analyses were performed using SPSS software program (version 22). Probability of P < 0.05 was regarded as significant at 95% confidence limit of significance between the two different groups of goats.
Results
Prevalence of the cysticercus ovis was tested among local and imported goats. The relevance of the months gives us a chance to study when is the highest rate of infection.
In table (1), it is recorded the number of infected and slaughtered local goats. The highest rate of infection (3.89%) among local goats was studied in the month of August 2017. And the lowest rate of infection (0.25%) recorded among the local goats was during the month of October 2018.
In table (2), the records are of the number of imported goats that were slaughtered and infected. The highest rate of infection among the slaughtered imported goats (5.30%) was recorded during the month of August 2017. The lowest rate of infected imported goats (1.72%) was recorded during the month of April 2018.
As shown in Table (3), the results showed significantly different prevalence of infection percentages, with no correlation between seasonal prevalence and mean temperature. The highest prevalence of cyctocercous ovis infection in goats was recorded during summer with 3.28% prevalence, whilst it was lowest during spring with 1.79% prevalence.
 |
Figure 1: Site of cysticercus ovis in goats collected from Al Kakee’s Slaughter, Makkah, Saudi Arabia (by Google map) |
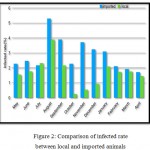 |
Figure 2: Comparison of infected rate between local and imported animals |
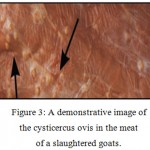 |
Figure 3: A demonstrative image of the cysticercus ovis in the meat of a slaughtered goats. |
Table 1: Number and infection rate of cysticercus ovis among slaughtered animals local.
| Year | Months | No. of slaughtered | No. of infected animals | Infection rate (%)* |
|
2017 |
May | 3336 | 52 | 1.55 |
| June | 3896 | 69 | 1.77 | |
| July | 2857 | 66 | 2.31 | |
| August | 5673 | 221 | 3.89 | |
| September | 9294 | 202 | 2.17 | |
| October | 2757 | 7 | 0.25 | |
| November | 2752 | 15 | 0.54 | |
| December | 2901 | 27 | 0.93 | |
| 2018
|
January | 3435 | 72 | 2.09 |
| February | 4060 | 71 | 1.74 | |
| March | 5325 | 94 | 1.76 | |
| April | 5414 | 79 | 1.45 | |
| Total | 51750 | 975 | 1.88 | |
*Infection rate= no of infected animals/ no. of slaughtered animals
Table 2: Number and infection rate of cysticercus ovis among slaughtered animals imported.
| Year | Months | No. of slaughtered | No. of infected animals | Infection rate (%) |
|
2017 |
May | 5202 | 119 | 2.28 |
| June | 4279 | 106 | 2.47 | |
| July | 3831 | 83 | 2.17 | |
| August | 6368 | 338 | 5.30 | |
| September | 8015 | 313 | 3.90 | |
| October | 3028 | 69 | 2.28 | |
| November | 3125 | 116 | 3.71 | |
| December | 3731 | 121 | 3.24 | |
| 2018
|
January | 4231 | 131 | 3.09 |
| February | 5354 | 113 | 2.11 | |
| March | 7162 | 138 | 1.92 | |
| April | 7535 | 130 | 1.72 | |
| Total | 61911 | 1777 | 2.87 | |
Table 3: Seasonal prevalence of cysticercus ovis infection in animals (* referred to highest infection percent).
| Infection rate (%) | No. of infected animals | No. of slaughtered | Season |
| 3.28* | 883 | 26904 | Summer |
| 2.44 | 722 | 29577 | Autumn |
| 2.25 | 535 | 23712 | Winter |
| 1.79 | 609 | 33974 | Spring |
Discussion
Cysticercus Ovis is a thin walled cyst that is filled with fluids and can be found in infected animals, causing their meat to be of threat if consumed by humans. The cyst in the wild domestic canids will degenerate over time and form a small nodule with a “gritty” texture which DeWolf et al., (2012) called sheep measles.
Cysticercus Ovis had infected 975 (1.88%) of locally raised goats in total throughout the year of the experiment. In imported goats, 1777 (2.87%) in total were found infected with the cysticercus ovis. Although the distribution of these cysts was found in different body parts and didn’t seem to follow any patterns, Minozzo et al., (2002) found the same result in his experiment.
The present investigation showed a high percentage of infection among slaughtered imported goats collected from Al kakee’s slaughterhouse in Makkah, Saudi Arabia. The highness percentage of infection was recorded to be 5.30% during the month of August in 2017. Our theory suggests that the results found were due to the fact that imported goats were suspected to be raised along with dogs or pigs, (unlike the local goats) as Smith & Sherman, 2009; Sharifiyazdi et al., (2011) agreed. The cysticercus ovis infects dogs and wild canids (such as Wolves and jackals) and cattle such as goats, sheep and camels and humans as their final host as agreed by Eckert & Deplazes, (2004). Deer can also be one of the intermediate hosts as reported by (Al-Sabi et al., 2013). Supports our theory is the recent study by Jenkins et al., (2018) in Australia where he found the presence of the T. Ovis parasite among 374,580 slaughtered goats which is believed is due to the fact that these goats live in areas where foxes and dingoes are present.
The highest level of infection in local goats were found during the month of August 2017 with a percentage of 3.89%, with that we conclude that the imported goats were infected more than the local goats collected. An observation that was proved by the tables and charts attached is that the highest level of infection amongst the two groups of goats was found during the warm months of summer which agrees with the researches that Opara et al., (2006) conducted on 25,800 cattle in Nigeria. This finding was also in agreement with the results Oryan et al., (1994) where he found the highest rate of infection was present in summer. Although, 11,228 cattle slaughtered in Ethiopia for an experiment conducted by Kebede et al., (2008) was found infected with a percentage of 7.5% with no relevance to the months of infection and 2.3 % of infection in sheep by Al Qureishy (2008) and 20% in goats from western Ethiopia by Sissay et al., (2008)
Our results all agreed with the results of Hashemnia et al., (2016) where they inspected 69,198 sheep in Iran and found the highest percentage of infection to be in spring with a number of 1.8%.
The results are due to numerous factors such as the high temperature of the summer months along with the wide spread of contagious diseases due to the weakness of the immune system. The weakness of the goat’s immune system makes widely spread parasites such as the cysticercus ovis easily contagious. Furthermore, easy access of animals to acquire infection with grass play is an important role in the epidemiology of this infection in the west of Iran as said by (Hashemnia et al., 2015).
In addition, 3% of infection was found among 500 inspected cattle in Iran by (Garedaghi., et al 2012), these results agree with the results we found among the slaughtered goats in Saudi Arabia.
Thus, we are capable of having a comparison regarding the cysticercus ovis infection between the imported and local goats, as to both groups were found with the highest percentage of infection during the summer months unlike any other time of the year. Many factors play a role in the results conducted such as the immune systems being weak during the summer months or an infection being acquired from grass.
The rate of the cysticercus ovis infecting humans has definitely increased if we compare the results found by (Sorvillo et al., 2007) (where 221 deaths were caused by cysticercosis over a 13 year study period) with the results found by Saini et al., (1997) (where he stated that the infection of the cysticercus ovis was “still rare in the united states”).
The contamination of the cattle meat causes a major economical loss as commented EARO, (2000) and Thompson & Lymbery, (1995) respectively, as this problem reduces the export earnings of the country, especially in extreme cases where the cattle die due to the disease as mentioned by Radfar et al., (2005) or when the infection rate is high like the results that Cabaret et al, (2002) found in Eastern Africa. Yet, countries such as china do not have enough data to implement disease control campaigns as researched by Zheng, (2016) regarding the Taenia ovis threatening the sheep of china. Although, some sites still hold a low rate of infection such as in western Canada as reported by (Lees et al., 2002). Therefore, currently many of the information we have about the cysticercosis is based on researches in New Zealand, Australia, Canada and some African countries. (Lawson 1994; DeWolf et al., 2012; Sissay et al., 2008)
It is as well a health hazard and a public catastrophe due to the fact that the aberrant host of the cysticercus ovis is humans, this causes Taeniasis. Symptoms vary from abdominal discomfort to loss of weight and many more as reported by Neva and Brown, (1994) depending on the organ infected in a human by the C.ovis. Therefore, these results calls for a higher level of inspection of slaughtered meat and an urgent therapeutic as well as a preventing program for this arising crisis.
Techniques such as disposing the infected meat of the dead sheep on farm by burning or burial so that they cannot be scavenged is an effective way to cut down on the spread of the infection as advised by (Anna Erickson, 2018). Unfortunately, the condemnation of an entire carcass is essential if numerous sheep were found infected as declared by (Hashemnia et al., 2016)
References
- Al-Sabi M. N. S., Chriél M., Holm E., Jensen T. K., Ståhl M & Enemark H. L. . Reappearance of Taenia ovis krabbei muscle cysts in a roe deer (Capreolus capreolus) in Denmark after 60+ years. Veterinary parasitology. 2013;196(1-2):225-229.
CrossRef - Al-Qureishy S. A. Prevalence of cestode parasites in sheep slaughtered in Riyadh City, Saudi Arabia. Journal of the Egyptian Society of Parasitology. 2008;38(1):273-280.
- Erickson A. Taenia ovis (Sheep measles) infection in sheep. Department of Agriculture and Food. 2018.
- Cabaret J., Geerts S., Madeline M., Ballandonne C & Barbier D. The use of urban sewage sludge on pastures: the cysticercosis threat. Veterinary research. 2002;33(5):575-597.
CrossRef - DeWolf B. D., Peregrine A. S., Jones-Bitton A., Jansen J. T., MacTavish J & Menzies P. I. Distribution of, and risk factors associated with, sheep carcass condemnations due to Cysticercus ovis infection on Canadian sheep farms. Veterinary parasitology. 2012;190(3-4):434-441.
CrossRef - Earo. Beef research strategy. Ethiopian Agricultural Research Organization, Animal Science Directorate, Addis Ababa. 2000.
- Eckert J & Deplazes P. Biological, epidemiological and clinical aspects of echinococcosis a zoonosis of increasing concern. Clinical microbiology reviews. 2004;17(1):107-135.
CrossRef - Garedaghi Y., Saber A. R & Khosroshahi M. S. Prevalence of bovine cysticercosis of slaughtered cattle in Meshkinshahr abattoir, Iran. Journal of animal and veterinary Advances. 2012.
CrossRef - Hashemnia M & Kish G. F. Prevalence and pathological lesions of ovine cysticercosis in slaughtered sheep in western Iran. Journal of parasitic diseases. 2016;40(4):1575-1578.
CrossRef - Hashemnia M., Shahbazi Y & Safavi E. A. A. Bovine cysticercosis with special attention to its prevalence, economic losses and food safety importance in Kermanshah, west of Iran. Journal of food quality and hazards control. 2015;2(1):26-29.
- Jenkins D. J., Cowled B & Brookes V. Taeniid metacestodes in rangeland goats in Australia. Veterinary parasitology. 2018;255:1-9.
CrossRef - Kebede N., Tilahun G & Hailu A. Current status of bovine cysticercosis of slaughtered cattle in Addis Ababa Abattoir, Ethiopia. Tropical animal health and production. 2009;41(3):291-294.
CrossRef - Lawson J. R. Hydatid disease and sheep measles: the history of their control and the economics of a recent change of control policy. New Zealand journal of zoology. 1994;21(1):83-89.
CrossRef - Less W., Nightingale J., Bromn D., Scandrett B & Gajadhar A. Outbreacof cysticercus bovis (Taenia saginata) in feedlot cattle in Alberata. Can Vet J. 2002;43:227-8.
- Minozzo J. C., Gusso R. L. F., Castro E. A. D., Lago O & Soccol V. T. Experimental bovine infection with Taenia saginata eggs: recovery rates and cysticerci location. Brazilian Archives of Biology and Technology. 2002;45(4):451-455.
CrossRef - Neghab M., Moosavi S & Moemenbellah-Fard M. D. Prevalence of intestinal parasitic infections among catering staff of students canteens at Shiraz, southern Iran. Pak J Biol Sci. 2006;9(14):2699-703.
CrossRef - Neva F. A & Brown H. W. Basic clinical parasitology (No. Ed. 6). Appleton & Lange. 1994.
- Sorvillo F. J., DeGiorgio C & Waterman S. H. Deaths from cysticercosis, United States. Emerging infectious diseases. 2007;13(2):230.
CrossRef - Opara M. N., Ukpong U. M., Okoli I. C & Anosike J. C. Cysticercosis of slaughter cattle in southeastern Nigeria. Annals of the New York Academy of Sciences. 2006;1081(1):339-346.
CrossRef - Oryan A., Moghaddar N & Gaur S. N. S. Metacestodes of sheep with special reference to their epidemiological status, pathogenesis and economic implications in Fars Province, Iran. Veterinary Parasitology. 1994;51(3-4):231-240.
CrossRef - Radfar M. H., Tajalli S & Jalalzadeh M. Prevalence and morphological characterization of Cysticercus tenuicollis (Taenia hydatigena cysticerci) from sheep and goats in Iran. Veterinarski arhiv. 2005;75(6):469.
- Saini P. K., Webert D. W & McCASKEY P. C. Food safety and regulatory aspects of cattle and swine cysticercosis. Journal of food protection. 1997;60(4):447-453.
CrossRef - Sharifiyazdi H., Oryan A., Ahmadnia S & Valinezhad A. Genotypic characterization of Iranian camel (Camelus dromedarius) isolates of Echinoccocus granulosus. The Journal of parasitology. 2011;97(2):251-255.
CrossRef - Sissay M. M., Uggla A & Waller P. J. Prevalence and seasonal incidence of larval and adult cestode infections of sheep and goats in eastern Ethiopia. Tropical Animal Health and Production. 2008;40(6):387-394.
CrossRef - Smith M. C & Sherman D. M. Liver and pancreas. Goat medicine. 2009;359-381.
CrossRef - Thompson R. A & Lymbery A. J. Echinococcus and hydatid disease. Cab International. 1995.
- Zheng Y. Taenia ovis: an emerging threat to the Chinese sheep industry? Parasites & vectors. 2016;9(1):415.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





