Manuscript accepted on : 10-October-2018
Published online on: 07-11-2018
Plagiarism Check: Yes
Evaluation of Antioxidant Activities of Alpinia galanga (L.) Willd
Khoirom Ratipiyari Devi1 , Paonam Priyobrata Singh2, Moirangthem Medhapati Devi1
, Paonam Priyobrata Singh2, Moirangthem Medhapati Devi1 and Gurumayum Jitendra Sharma1
and Gurumayum Jitendra Sharma1
1Department of Life Sciences, Manipur University, Imphal-795003, India.
2Pandit Deen Dayal Upadhyay Institute of Agricultural Sciences, Utlou - 795134, India.
Corresponding Author E-mail: ratikhoirom@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2700
ABSTRACT: Present research was designed to evaluate the free radical scavenging capacities and antioxidant activities of rhizome extracts of Alpinia galanga prepared in different solvent systems (60% aqueous methanol, 60% aqueous ethanol and distilled water) using different in vitro chemical assays. Antioxidant components such as total phenolic content (TPC), total flavonoid content (TFC) and ascorbic acid contents of the ginger species were screened. Antioxidant assays employed included sulphur free radical reactivity assay, ferric ion reducing power assay, DPPH free radical scavenging capacity assay, hydroxyl radical scavenging assay, nitric oxide scavenging activity assay and hydrogen peroxide scavenging assay. The obtained data reveal that the plant extracts contained significant amount of the observed antioxidant components and also exhibited significant free radical scavenging capacities. Methanol (60%) extract exhibited highest antioxidant activity than other solvents. The polyphenolic constituents of the plant extracts appear to be largely responsible for the radical scavenging capacity. The plant extracts act as promising source of antioxidants, and may be useful for development of nutraceuticals and pharmaceutical drugs.
KEYWORDS: Alpinia Galangal; Antioxidant; Free Radicals; Phytochemicals; Reactive Oxygen Species (ROS)
Download this article as:| Copy the following to cite this article: Devi K. R, Singh P. P, Devi M. M, Sharma G. J. Evaluation of Antioxidant Activities of Alpinia galanga (L.) Willd. Biosci Biotech Res Asia 2018;15(4). |
| Copy the following to cite this URL: Devi K. R, Singh P. P, Devi M. M, Sharma G. J. Evaluation of Antioxidant Activities of Alpinia galanga (L.) Willd. Biosci Biotech Res Asia 2018;15(4). Available from: https://www.biotech-asia.org/?p=31685 |
Introduction
Free radicals and ROS are highly reactive atoms or group of atoms produced constantly in our body during normal metabolic functions or introduced from the environment such as exposure to solar radiation, air pollution, ionizing radiations and smoking. Overproduction of free radicals may results into oxidative stress, which contributes to more than hundred disorders in human including hypertension, atherosclerosis, rheumatoid arthritis, diabetes mellitus, stroke, myocardial infarction, reperfusion injury, gastritis, Parkinson’s disease, haemorrhagic shock, coronary heart diseases, neuro-degeneration, cataract, carcinogenesis, inflammatory disorders, AIDS as well as age-related brain disorders.1,2 Antioxidants present in plants play vital role in neutralizing free radicals and oxygen-derived species by interfering with oxidation process, chelating catalytic metals and also act as oxygen scavengers.3 ROS, superoxide anion, hydroxyl radical and hydrogen peroxide that are generated as by-products of biological reactions or from exogenous factors react with nearly every molecule found in living cells including DNA, if excess ROS are not eliminated by antioxidant system. Since many antioxidants such as citric acid, propyl gallate, butylated hydroxyanisole (BHA), and butylated hydroxytoluene (BHT) are reported to have many side effects including toxicity and cause lipid alteration as well as carcinogenesis,4,5 attention is being focussed on the development of new, safe and non-toxic, economic and powerful natural antioxidants.
Plants are important source of phytochemicals with potential therapeutic effects. Presence of a diverse group of phytochemicals such as phenolic acids, flavonoids, steroids, glycosides, alkaloids, tannins, lignins, stilbenes, anthocyanins etc. contribute to the biological activities of plants including anti-oxidant activity.6,7 Phenolic compounds or polyphenols are a group of heterogeneous plant secondary metabolites with an aromatic ring and are ubiquitously distributed in the plant kingdom. Flavonoids are also widely distributed in almost all plant families and they easily scavenge aqueous free radicals because of their amphipathic characteristics.8 This group of compounds have potential to inhibit ROS, damage by haem protein/peroxide mixtures and lipoxygenase and cyclooxygenase enzymes in vitro.9
Alpinia galanga (L.) Willd. (commonly called greater galanga) is a perennial aromatic rhizomatous herb of Zingiberaceae family which is widely cultivated in South East Asia. The plant is widely used as spices for flavouring food as well as herbal remedy in traditional system of medicine such as Ayurveda, Unani, Chinese and Thai folk medicine.10 The rhizome has a wide range of application as herbal medicine in the treatment of rheumatoid arthritis, ulcer, whooping colds in children, asthma, bronchitis, intermittent fever, stomachache and colic, vomiting, indigestion.11,12 It is also used as remedy for halitosis, dyspepsia, diabetes and sexual impotency. Phytochemical constituents of the plants have been reported to possess anti-inflammatory, analgesic, antifungal, antibiotic, antibacterial, antiulcer, and anticancer properties.13,14,12,15 These multiple pharmacological and medicinal properties of Alpinia galanga have triggered our interest to evaluate its antioxidant potential. In the present study, antioxidant activities were assessed using sulphur free radical reactivity assay, ferric ion reducing power assay, DPPH free radical scavenging capacity assay, hydroxyl free radicals scavenging assay, nitric oxide scavenging activity assay and hydrogen peroxide scavenging activity assay. Antioxidant components such as total phenolic content, total flavonoid content and ascorbic acid contents were also estimated.
Materials and Methods
Reagents and Chemicals
Ascorbic acid, curcumin, Folin-Ciocalteu, TCA, DPPH, glutathione and quercetin were obtained from Sigma-Aldrich, USA. FeSO4, Sodium carbonate, gallic acid, potassium hexacyanoferrate, sodium salicylate, metaphosphoric acid, naphthylethylenediamine dihydrochloride, Na2-EDTA, trichloroacetic acid, and nitroblue tetrazolium (NBT) were procured from HiMedia, India. Other chemicals and reagents were of analytical grade obtained from Merck, India.
Preparation of Extract
Fresh rhizomes of mature plants of Alpinia galanga (L.) Willd. were collected and washed thoroughly in tap water. Outer scales of rhizomes were removed by a sharp scalpel. One gram dry weight equivalent of fresh rhizomes relative to the moisture content was ground using mortar and pestle, and the paste was treated with 10 ml of 60% aqueous methanol, distilled water, or 60% aqueous ethanol and homogenized separately for each extract. The homogenate was collected and centrifuged at 3000 X g for 10 min to get a clear supernatant. Finally, the clear supernatant was decanted and filtered through Whatman No. 1 filter paper and stored at 4°C.
Determination of Antioxidant Components
Antioxidant phytochemicals such as total phenolics, total flavonoids and ascorbic acid content were estimated as per the procedures of our earlier studies16,17,18. Total phenolic content was expressed as gallic acid equivalents (GAE) in mg.100g-1. The concentration range of gallic acid used for standard curve was 2-16µg.ml-1, and the equation of standard gallic acid curve was y = 0.0757x + 0.0443 (r2= 0.9926). For the estimation of total flavonoid content, quercetin at the concentration range of 5 to 100 µg.ml-1 was used as a standard compound and the equation of the calibration curve was y = 0.0074x + 0.0019 (r2 = 0.9974). For the measurement of ascorbic acid content, the concentration range of ascorbic acid used for the construction of calibration curve was 0.32-3.52 µg.ml-1 and equation of the calibration curve was found to be y = – 0.2535 x + 0.9755 (r2=0.9879).
Determination of Antioxidant Potentials
Potential of the plant extract to scavenge free radicals were determined through sulphur / thiyl free radical reactivity assay,19 ferric ion reducing power assay, DPPH free radical scavenging capacity assay, hydroxyl radicals scavenging assay, nitric oxide scavenging activity assay and hydrogen peroxide scavenging assays following the methods published elsewhere.20,21,22
Results and Discussions
Determination of Antioxidant Components
Total Phenolic Content
Phenolic compounds are secondary metabolites possessing high antioxidant properties that are derived from the pentose phosphate, shikimate and phenylpropanoid pathways in plants.23 Their antioxidant activity is mainly due to their redox properties which allow them to act as radical scavengers, metal chelators, reducing agents, hydrogen donors, and singlet oxygen quenchers. Total phenolic content (TPC) of the plant extracts was expressed as gallic acid equivalent (GAE) in 100g of sample. 60% methanol extract showed highest TPC than that of 60% ethanol and aqueous extracts (Table 1). TPC of 60% methanol, 60% ethanol and aqueous extracts of the plant were found to be 184.52 ± 0.41, 152.96 ± 0.57 and 116.38 ± 0.38 mg GAE.100g-1 respectively.
Total Flavonoid Content
Flavonoids are secondary metabolites with scavenging properties against most oxidizing molecules, including singlet oxygen, and various other free radicals implicated in several diseases.24 They possesses therapeutic potential, including cardioprotective, anti-inflammatory, antimicrobial, and antitumor.25 Potential health benefits of flavonoids are due to their antioxidant activities which can be attributed to the phenolic hydroxyl groups attached to the flavonoid structure.26 Hydroxyl group present in the molecular structure of flavonoids could donate an electron (H+) to radicals such as hydroxyl (HO•), superoxide (O2•) and peroxyl (ROO•) and thus neutralizing them.27 Total flavonoid content (TFC) of the plant extracts were expressed as quercetin equivalent (QE) in 100g of sample. 60% methanol extract showed highest TFC than that of 60% ethanol and aqueous extracts. TFC of 60% methanol, 60% ethanol and aqueous extracts of the plantwere found to be 278.15 ± 0.42, 234.54 ± 0.63 and 203.86 ± 0.47 mg QE.100g-1 respectively (Table 1).
Ascorbic Acid Content
Ascorbic acid acts as a powerful antioxidant in fighting free-radical mediated diseases. It can regenerate other antioxidants within the body, including alpha-tocopherol or vitamin E.28,29 Unlike to most other animals, human are not able to synthesize ascorbic acid. So, it must be obtained from diet and, must be obtained regularly as it cannot be stored in the body. Ascorbic acid is an excellent source of electrons and directly react with or neutralize hydroxyl, alkoxyl and lipid peroxyl (ROO.) radicals as well as radical form of other antioxidants such as glutathione radical and vitamin E radical, thereby regenerating these antioxidants.30 Due to soluble in water, it can combat free radical damages both inside and outside the cells. Ascorbic acid content as determined from the calibration curve was found to be 35.28 ± 0.36, 48.74 ± 0.41 and 54.19 ± 0.57 mg.100-1g for 60% methanol, 60% ethanol and aqueous extracts respectively (Table 1). The antioxidant activity of rhizome might be attributed to its phenolic compounds, flavonoids and ascorbic acid contents.
Table 1: Antioxidant components of the rhizome extracts of in different solvent systems.
| Solvent type for plant extract | Total phenolic content
(mg GAE.100g-1) |
Total flavonoid content
(mg QE.100g-1) |
Ascorbic acid content
(mg.100g-1) |
| 60% Methanol | 184.52 ± 0.41 | 278.15 ± 0.42 | 35.28 ± 0.36 |
| 60% Ethanol | 152.96 ± 0.57 | 234.54 ± 0.63 | 48.74 ± 0.41 |
| Aqueous | 116.38 ± 0.38 | 203.86 ± 0.47 | 54.19 ± 0.57 |
Values are expressed as mean ± S.D. (n=3). Values are statistically significant at p ≤ 0.05 as determined by Duncan’s test. ANOVA does not apply between columns.
Sulphur / Thiyl Free Radical Reactivity Assay
Thiyl free radicals (GS˙) are formed in gamma irradiated aqueous solution of glutathione. Oxidation of curcumin due to GS˙ results in the depletion of chrome orange-yellow compound curcumin. Antioxidant potentials of rhizome extract in terms of thiyl free radical scavenging capacity was determined by their ability to protect curcumin from sulfur free radicals. A substantial depletion of curcumin was observed when the reaction mixture was supplemented with different doses of plant extracts (200, 500 and 700 µL) (Fig. 1). Inhibition of curcumin depletion was found to increase with increasing extract dose which indicates that the plant possess bioactive molecules having antioxidant properties thereby protecting the curcumin molecules present in the reaction mixture by scavenging thiyl free radicals. The crude rhizome extract at the concentration of 6 mg.ml−1 inhibited curcumin depletion in the reaction mixture at 60 Gy radiation exposure to the extent of 3.18 μM. Under the same treatment of radiation exposure, these values were changed to 1.72 μM. at the extract concentration of 16 mg.ml−1,which is further shifted to 1.08 μM at the extract concentration of 24 mg.ml.−1 Similar trend in the protection of curcumin depletion were observed in other cases of radiation exposure. Protective Index was found to be low at the lower dose of 60 Gy, and increased until the dose reached 240 Gy but declined with further increase in radiation dose (Fig. 2). Increase in PI at lower radiation dose can be due to formation of lesser population of thiyl free radicals that were able to compete with curcumin, whereas after a threshold dose, thiyl free radical generation increases, resulting in greater damage, causing depletion in curcumin.
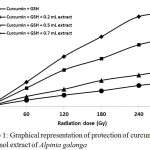 |
Figure 1: Graphical representation of protection of curcumin by 60% methanol extract of Alpinia galanga.
|
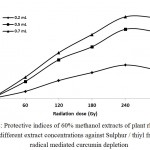 |
Figure 2: Protective indices of 60% methanol extracts of plant rhizomes at different extract concentrations against Sulphur / thiyl free radical mediated curcumin depletion.
|
Fe3+ Reducing Power Assay
For the measurement of the reducing ability, the reduction of Fe3+ → Fe2+ was analysed with the plant extracts. Antioxidants are able to donate electrons to reactive radicals, reducing them into more stable and unreactive species. The production of Perl’s Prussian blue colouration due to the formation of Fe2+ can be monitored at absorbance of 700 nm by spectrophotometer. The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity. However, the activity of antioxidants has been assigned to various mechanisms such as prevention of chain initiation, binding of transition metal ion catalysts, decomposition of peroxides, prevention of continued hydrogen abstraction, reductive capacity and radical scavenging. Basically, reducing power is associated with the presence of reductones that break the free radical chain by donating a hydrogen atom.31 Antioxidants present in the plant extracts can donate electrons to free radicals, which lead to the neutralization of the radicals. Fig. 3 shows the reductive ability of the crude extracts of the plant rhizomes. The reducing power of plant extracts was increased with increased dosage.
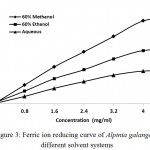 |
Figure 3: Ferric ion reducing curve of Alpinia galanga in different solvent systems DPPH free Radical Scavenging Capacity Assay.
|
DPPH assay has been widely used to evaluate the free radical scavenging capacity of the plant extracts or their compounds as it is simple, rapid and highly sensitive. Due to the presence of an odd electron, this nitrogen-centred radical gives a maximum absorption at about 515 nm and accepts an electron from an antioxidant compound which acts as a hydrogen donor. The scavenging activity of the plant extracts was monitored based on the amount of DPPH radicals remaining in the test sample using a spectrophotometer. Ascorbic acid was used as standard compound because it impairs the formation of free radicals in the process of intracellular substance formation throughout the body. The purple coloured solution of DPPH free radical was decolorized to its yellow coloured hydrazine, when supplemented with different concentrations of plant extracts to the 100 μM solution of DPPH. The degree of decoloration increased with increasing concentration of the plant extracts. Rhizome extracts in different solvent systems (aqueous, 60% ethanol and 60% methanol) showed good scavenging capability against DPPH free radical. At extract concentration of 18 mg/ml, plant extracts inhibited 81.75%, 86.61% and 92.4% for aqueous, 60% ethanol and 60% methanol extracts respectively (Fig. 4). The highest scavenging activity was observed in the plant extracts made in 60% methanol with an IC50 of (7.22 ± 0.57 mg/ml). This is followed by the 60% ethanol extract (8.54 ± 0.38 mg/ml) and water fraction (9.33 ±0.46 mg/ml) (Table 2). Ascorbic acid, used as standard compound for this experiment has IC50 5.07µg.ml-1.
Table 2: Free radical scavenging capacities through different antioxidant assays using different solvent systems expressed as IC50.
| Type of solvent | DPPH assay
IC50 (mg/ml) |
Hydroxyl
IC50 (mg/ml) |
Nitric
IC50 (mg/ml) |
Hydrogen peroxide
IC50 (mg/ml) |
| 60% Methanol | 7.22 ± 0.57 | 4.37 ± 0.43 | 0.61 ± 0.46 | 1.87 ± 0.39 |
| 60% Ethanol | 8.54 ± 0.38 | 5.54 ± 0.61 | 0.69 ± 0.61 | 2.29 ± 0.56 |
| Aqueous | 9.33 ± 0.46 | 7.06 ± 0.54 | 0.82 ± 0.42 | 2.74 ± 0.42 |
Values are expressed as mean ± S.D (n=3). Values are statistically significant at p ≤ 0.05 as determined by Duncan’s test. ANOVA does not apply between columns.
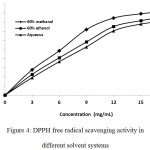 |
Figure 4: DPPH free radical scavenging activity in different solvent systems.
|
Hydroxyl Radical Scavenging Assay
Hydroxyl radical is known to be the most powerful oxidizing radical of all the reduce forms of dioxygen and responsible for the oxidative damage of most biomolecules in living cells, such as sugars, proteins, DNA, polyunsaturated fatty acid in membranes and most of the biological molecules it contacts and are capable of abstracting hydrogen atoms from membrane lipids through peroxidation reaction of lipids.32,33 It generates various products from the DNA bases which mainly include C-8 hydroxylation of guanine to form 8-oxo-7, 8 dehydro- 2 deoxyguanosine, a ring opened product.34 Thus, removal of OH radical is very important for the protection of living systems. In the present study, the plant extracts exhibited a concentration dependent scavenging activity against hydroxyl radicals generated. 60% methanolic extracts of plant rhizomes showed relatively maximum degree of scavenging activity towards hydroxyl free radicals than 60% ethanol and aqueous extracts. The degree of inhibition increased with increasing concentration of the extracts. At extract concentration of 24 µg/ml, it exhibited 87.57%, 93.58% and 97.62% inhibition for aqueous, 60% ethanol and 60% methanol extracts respectively (Fig. 5). IC50 of this assay were found to be 4.37 ± 0.43, 5.54 ± 0.61 and 7.06 ± 0.54 mg/ml for extracts dissolved in 60% methanol, 60% ethanol and distilled water respectively (Table 2). IC50 of curcumin for this experiment was found to be 26.36 µg.ml.-1
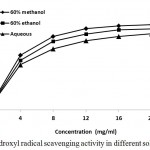 |
Figure 5: Hydroxyl radical scavenging activity in different solvent systems.
|
Nitric Oxide Scavenging Activity Assay
NO radical plays a multiple role in diverse biological systems including an effector molecule, neuronal messenger, vasodilatation and antimicrobial as well as antitumor activities.35 Inhibitors of nitric oxide are known to be beneficial on some aspect of inflammation and tissue damages associated with inflammatory diseases. Nitric oxide radical scavenging assay is based on the generation of nitric oxide from sodium nitroprusside in buffered saline, which reacts with oxygen to produce nitrite ions which can be measured by using Griess reagent.36 Incubation of solutions of sodium nitroprusside in phosphate buffer saline at 25°C for 150 min resulted in the generation of NO. The plant extracts effectively reduced the generation of NO as indicated by the significant decreased in the absorbance of the reaction mixtures with increasing amount of plant extracts. Fig. 6 illustrates a significant decrease in the NO radical due to the scavenging activity of plant extracts. 60% methanolic extract showed maximum percentage of scavenging activity, followed by 60% ethanolic and aqueous extracts. At extract concentration of 1.5 µg/ml, it showed 68.45%, 73.51% and 78.24% inhibition for aqueous, 60% ethanol and 60% methanol extracts respectively. Lowest IC50 values for both samples were observed in case of 60% methanolic extracts as compare to 60% ethanolic and aqueous extracts (Table 2). IC50 were found to be 0.61 ± 0.46, 0.69 ± 0.61 and 0.82 ± 0.42 mg/ml for plant extracts dissolved in 60% methanol, 60% ethanol and distilled water respectively. Ascorbic acid has IC50 of 7.90 µg ml-1 for this experiment.
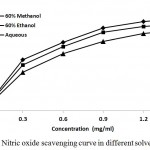 |
Figure 6: Nitric oxide scavenging curve in different solvent systems.
|
Hydrogen Peroxide Scavenging Assay
Hydrogen peroxide can be produced either in biological systems or through in vivo by several oxidizing enzymes such as superoxide dismutase. It is a weak oxidizing agent and can cross membranes which may results into oxidation of a number of compounds. For instance, it deactivates the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase37. It is toxic and induces cell death in vitro. It is used in the respiratory burst of activated phagocytes.38 In our study, the ability of plant extracts to scavenge hydrogen peroxide is shown in Fig. 7. Here, plant extracts of different solvents showed hydrogen peroxide decomposition activity in a concentration dependent manner. At extract concentration of 5 mg /mL, it exhibited 71.22%, 82.19% and 90.88% inhibition for plant extracts dissolved in distilled water, 60% ethanol and 60% methanol respectively (Fig.7). Maximum scavenging activity was observed in case of 60% methanolic extracts as indicated by its lowest IC50 value, followed by 60% ethanol and aqueous extracts (Table 2). IC50 were found to be 1.87 ± 0.39, 2.29 ± 0.56 and 2.74 ± 0.42 mg.mL-1 respectively for extracts dissolved in 60% methanol, 60% ethanol and distilled water respectively.
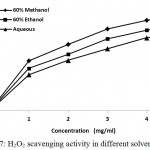 |
Figure 7: H2O2 scavenging activity in different solvent systems.
|
Conclusion
The results obtained in the present study indicate that rhizome extract of Alpinia galanga used both as non-conventional food plant and also as herbal medicine exhibit significant free radical scavenging and reducing power activity. The rhizome extract prepared in 60% methanol exhibit highest antioxidant activity as compared to the other rhizome extracts which might be attributed to its phytochemical constituents including phenolic and flavonoid compounds. The findings of the present study demonstrate that Alpinia galanga rhizome can be a noteworthy resource of natural antioxidant as can be vouched from the efficient free radical scavenging activities with possible applications in pharmacology and medicine for countering the oxidation of cells in the human body caused by free radicals. Supplementation of this non-conventional rhizomatous food plant in the dietary intakes would significantly help in enhancing cellular free radical defensive processes thereby protecting cells from undergoing subsequent damages and possible neoplastic transformations.
Acknowledgement
The authors are thankful to CSIR- HRD Group, Government of India, New Delhi, India for providing financial support (vide Office order / File No. 09/476 (0078)/2017–EMR-I, dated 3rd May, 2017). G J S is thankful to the University Grants Commission, Government of India for financial support [vide No. F.18-1(103)/2017 (BSR)].
Conflict of Interest
There is no conflict of interest.
Funding Sources
This study was supported by the financial grant provided by CSIR- HRD Group, Government of India, New Delhi, India (vide Office order / File No. 09/476 (0078)/2017–EMR-I, dated 3rd May, 2017). Further, G J S is also thankful to the University Grants Commission, Government of India for financial support [vide No. F.18-1(103)/2017 (BSR)].
References
- Wadkar K., Magdum C. S. Evaluation of total phenolic content, flavonoid content and antioxidant activity of stem bark of Careya arbores Roxb. J . Pharmacogn. Phytochem. 2010;2(1):49-51.
- Wang Y. F., Wang , Wu J., Xu P., Wang Y. Q., Gao J. J., Hochstetter D. In vitro antioxidant activity and potential inhibitory action against α-glucosidase of polysaccharides from fruit peel of tea (Camellia sinensis L.). J. Zhejiang Univ. Sci. B. 2014;15:173-180.
CrossRef - Ramalingam S., Devarajan N., Muthugounder S. S. Antioxidant compound quercetin-3-O-α-L-rhamnoside(1→6)-β-D-glucose (Rutin) isolated from ethyl acetate leaf extracts of Memecylon edule (Melastamataceae). Free Radic. Antiox. 2015;5:35-42.
CrossRef - Grillo C. A., Dulout F. N. Cytogenetic evaluation of butylated hydroxytoluene. Res. 1995;345:73-8.
CrossRef - Rice-Evans C. A., Packer L. (ed): Flavonoids in Health and Disease, 2nd New York: CRC Press Inc, Bosa Roca, USA. 2003.
- Boligon A. A., Schwanz T. G., De Brom T. F., Frohlich J. K., Nunes L., Mario, D. M. Chemical composition, antioxidant and antimicrobial activity of the essential oil of Scutia buxifolia Reissek leaves. Anal. Acta. 2012;3(10):1-4.
- Yang Z., Liu C., Xiang L., Zheng Y. Phenolic alkaloids as a new class of antioxidants in Portulacaoleracea. Res. 2009;23(7):1032-5.
- Riou V., Vernhet A., Doco T., Moutounet M. Aggregation of grape seed tannins in model wine effect of wine polysaccharides. Food Hydrocoll. 2002;16:17-23.
CrossRef - Read M. A. Flavonoids: naturally occurring anti-inflammatory agents. J. Pathol. 1995;147:235.
- Yang X., Eilerman R. G. Pungent principle of Alpinia galanga (L.) Swartz and its application. Agric. Food Chem. 1999;47:1657-1662.
CrossRef - Burkill I. H. (ed): A Dictionary of the Economic Products of the Malay Peninsula. London: Crown Agent. 1966.
- Matsuda H., Pongpiriyadacha Y., Morikawa T., Ochi M., Yoshikawa M. Gastroprotective effects of phenylpropanoids from the rhizomes of Alpinia galanga in rats: structural requirements and mode of action. J. Pharmacol. 2003;471(1):59-67.
CrossRef - Borthakur M., Hazarika J., Singh R. S. A protocol for micropropagation of galanga. Plant Cell. Tiss. Organ Cult. 1999;55:231-233.
CrossRef - Juntachote T., Berghofer E., Siebenhandl S., Bauer F. The antioxidative properties of holy basil and galangal in cooked ground pork. Meat Sci. 2006;72 (3):446-456.
CrossRef - Thomas E., Shanmugan J., Rafi M. M. Antibacterial activity of plants belonging to Zingiberaceace family. BioMedicine. 1996;16:15-20.
- Moirangthem M. D., Paonam P.S., Khoirom R.D., Thokchom D.S., Sharma G. J. In-vitro free radical scavenging activity and radioprotective property of Zingiber kangleipakense (Kishor & Škorničk). J. Pharmacogn. Phytochem. Res. 2016;8(1):135-142.
- Paonam P., Thokchom D.S., Sharma G. J. Free radical scavenging and antioxidant potentials of Panax pseudoginseng J. Free Radic. Antiox. 2013;139:289-294.
- Thokchom D. S., Sharma G. J. Free radical scavenging activity of some therapeutic plants and protection of radiation-induced DNA damage by Zingiber montanum J. Herbs Spices Med. Plants 2012;18(1):1-17.
CrossRef - Aquino D’., Bullion C., Chopra M., Devi D., Devi S., Dunster C., James G., Komuro E., Kundu S, Niki E., Raza F., Robertson F., Sharma J., Willson R. Sulfhydryl free radical formation enzymatically, by sonolysis, by radiolysis and thermally: vitamin A, curcumin, muconic acid, and related conjugated olefins as references. Methods 1994;233:34-46.
- Paonam P., Khoirom R., Moirangthem M., Sharma G. J. Comparative analysis of antioxidant activities and screening of certain bioactive compounds from rhizome extracts of Paris polyphylla and Panax pseudognseng. In: Current Topics in Redox Biology (Sharma GJ, Sharan RN, ed). New Delhi: McGraw Hill Education. 2014; pp 255- 267.
- Paonam P.S., Khoirom R. D., Moirangthem M. D., Thokchom D. S., Sharma G. J. Protection of low LET radiation-induced DNA damage in rat bone marrow cells by free radical scavenger curcumin. Int J. Pharm. Sci. Res. 2016;7(3):1168-1178.
- Sharma G. J. Tropical Ginger Extract as Protectant in Radiation Countermeasures . Trad. Med. 2017; 3:123-135.
CrossRef - Randhir R., Lin Y. T., Shetty K. Phenolics, their antioxidant and antimicrobial activity in dark germinated sprouts in response to peptide and phytochemical elicitors. Asia Pac. J. Clin. Nutr. 2004;13:295–307.
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism and nutritional significance. Rev. 1998.56:317-333.
- Akhlaghi M., Bandy B. Mechanisms of flavonoid protection against myocardial ischemia-reperfusion injury. Mol. Cell. Cardiol. 2009;46:309-317.
CrossRef - Kanner J., Frankel E., Granit R., German J. B., Kinsella J. E.. Natural antoxidants in grapes and wines. Agric. Food Chem. 1994;42:64‐69.
CrossRef - Harborne J. B., Williams C. A. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481-504.
CrossRef - Davey M. W., Monatgu M. V., Sanmatin M., Kanellis A. S., Benzie J. J., Strain J. J., Favell D., Fletcher J. Plant l-ascorbic acid chemistry function metabolism bioavailability and effects of processing. J. Sci. Food Agric. 2000;80:825–860.
CrossRef - Jacob R. A., Sotoudeh G. Vitamin C function and status in chronic disease. Clin. Care. 2002;5:66-74.
CrossRef - Lu J. M., Lin P. H., Yao Q. Z., Chen C. Y. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. Cell Mol. Med. 2010;14(4):840–60.
CrossRef - Duh P. D. Antioxidant activity of burdock (Arctium lappa Linne): its scavenging effect on free radical and active oxygen. Am. Oil Chem. Sci. 1998;75:455–461.
CrossRef - Nabavi S. M. In vitro antioxidant and free radical scavenging activity of Diospyros lotus and Pyrusboissieriana growing in Iran. Mag. 2009;4(18):123-127.
- Rollet-Labelle E., Grange M. J., Elbim C., Marquetty C., Gougerot-Pocidalo M. A., Pasquier C. Hydroxyl radical as a potential intracellular mediator of polymorphonuclear neutrophil apoptosis. Free Radic. Biol. Med. 1998;24(4):563–572.
CrossRef - Tsuboi H., Kouda K., Takeuchi H., Takigawa M., Masamoto Y., Takeuchi M. 8-hydroxydeoxyguanosine in urine as an index of oxidative damage to DNA in the evaluation of atopic dermatitis. J. Dermatol. 1998;138:1033–5.
CrossRef - Hagerman A. E., Riedl K. M., Jones G. A., Sovik K. N., Ritchard N. T., Hartzfeld P.W. High molecular weight plant polyphenolics (tannins) as biological antioxidants. Agric. Food Chem. 1998;46:1887-1892.
CrossRef - Miller N. J., Rice-Evans C. A., Davies M. J., Gopinathan V., Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993;84:407-412.
CrossRef - Hyslop P. A., Hinshaw D. B., Halsey W. A. Mechanisms of oxidantmediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. Biol. Chem. 1988;263:1665–1675.
- Mac Donald-Wicks L. K., Wood L.G., Garg M. L. Methodology for the determination of biological antioxidant capacity in vitro: a review. J. Sci. Food Agric. 2006;86:2046- 2056.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





