Manuscript accepted on : 17-Sep-2018
Published online on: 26-09-2018
Plagiarism Check: Yes
Akpomie K. G1 , Ezeofor C. C1, Eze S. I1, Okey C. N1 and Ebiem-Kenechukwu P. I2
, Ezeofor C. C1, Eze S. I1, Okey C. N1 and Ebiem-Kenechukwu P. I2
1Department of Pure and Industrial Chemistry, University of Nigeria, Nsukka, Nigeria.
2Projects Development Institute (PRODA), Emene, Enugu, Nigeria.
Corresponding Author E-mail: eze.samson@unn.edu.ng
DOI : http://dx.doi.org/10.13005/bbra/2663
ABSTRACT: The biosorption of Cd (II), As (III) and Pb (II) ions from solution utilizing Vigna unguiculata leaf powders (VULP) as a low cost biosorbent was studied. The influence of temperature, metal ion concentration, biosorbent dose, contact time and pH on the sequestration process was examined by batch procedure. Increase in the biosorption of the three metal ions with increased pH and biosorbent dosage was obtained in this study.Equilibrium contact time of 20, 40 and 50min was achieved for Cd(II), As (III) and Pb(II) ions and biosorption was in the order As(III)> Cd(II) >Pb(II). Isotherm analysis was performed by the application of Langmuir, Freundlich, Flory-Huggins and Scatchard models. The Langmuir model gave the best fit with maximum monolayer biosorption capacity of 109.1, 105 and 119.3 mg/g for Cd (II), Pb (II) and As (III) respectively. Scatchard model confirmed a homogenous surface of VULP and monolayer biosorption of metal ions. Pseudo second order model showed the best fit compared to pseudo first order, Elovich and Banghams kinetic models according to kinetic analysis. Thermodynamics study revealed a feasibly, spontaneous exothermic biosorption process. The result showed good potentials of VULP as suitable cheap biosorbent for attenuation of Cd (II), Pb(II) and As (III) ions from polluted wastewaters.
KEYWORDS: Arsenic (III); Biosorption; Isotherm; Pollution; Vigna unguiculata; Wastewater
Download this article as:| Copy the following to cite this article: Akpomie K. G, Ezeofor C. C, Eze S. I, Okey C. N, Ebiem-Kenechukwu P. I. Biosorptive Removal of Lead (II), Cadmium (II) and Arsenic (III) from Aqua Media on Vigna Unguiculata Leaf Powders. Biosci Biotech Res Asia 2018;15(3). |
| Copy the following to cite this URL: Akpomie K. G, Ezeofor C. C, Eze S. I, Okey C. N, Ebiem-Kenechukwu P. I. Biosorptive Removal of Lead (II), Cadmium (II) and Arsenic (III) from Aqua Media on Vigna Unguiculata Leaf Powders. Biosci Biotech Res Asia 2018;15(3). Available from: https://www.biotech-asia.org/?p=31140 |
Introduction
Due to increasing development in science and technology, the number of applications of metals for commercial purposes has continued to increase. As a result, large amount of metallic waste are generated from industries and discharged in water bodies as effluents. The harmful effect of heavy metals discharged annually into the environment exceeds greatly the toxicity of all radioactive and organic wastes combined together.1 This is the reason why the discharge of heavy metals from effluents into the environment is a problem of great concern over the decades. The industrial activities responsible for the discharge of heavy metals into our surrounding include leather tanning, battery manufacturing, electroplating, metal finishing, steel fabrication, paint production, ceramics, glass, dyes and paper production. Heavy metals such as chromium, cadmium, lead, arsenic, copper, nickel, and mercury are known to be harmful at certain concentrations.2 The contamination of the environment with Lead is mainly due to anthropogenic activities, which makes this metal the most ubiquitous toxic metal in the environment. Lead is non-biodegradable, has the potential to bio-accumulate in the food chain causing human health hazards. Also, when present in high concentrations can damage the brain and nervous system.3 The assimilation of relatively small amounts of Lead in humans can lead to chronic toxicity and malfunctioning of the organs. Lead is also an enzyme inhibitor, general metabolic poison and affects the functioning of the blood, liver and kidney. Cadmium is used in most chemical industries for manufacturing pesticides, herbicides and fungicides. It has been reported that cadmium is highly toxic because of its lack of homeostatic control in the human body. Retention of ingested cadmium at the level of about 1-2% in the body is very harmful to human health. It is an enzyme inhibitor and is responsible for kidney tubular impairment, affects calcium metabolism, skeletal calcification and ion regulation. It has been reported to cause diarrhea, vomiting, a choking sensation, severe abdominal pain and liver damages.3 Arsenic is a known toxic element; arsenic (III) is more poisonous than arsenic (V) because of its binding to single but with higher affinity to functional groups that reacts with different protons thereby inhibiting their activity. Prolonged intake of drinking water contaminated with arsenicgives rise to kidney, skin and lungs cancer, skin, cardiovascular diseases, bone marrow disorder, gastrointestinal disease and other diseases.4 As a result of the magnitude of the problem resulting from heavy metal pollution, several research works on the elimination of these metals from effluents have become a topic of interest for environmental scientist. The traditional techniques which were used for the elimination of heavy metals from industrial wastewaters include filtration and chemical precipitation, evaporation, solvent extraction, reverse osmosis, ion exchange, electrochemical treatment and reduction or chemical oxidation.The disadvantage of these techniques include low selectivity, high cost , incomplete metal recovery, increased energy requirement, difficult to apply and the formation of harmful slurries which are strenuous to dispose.5 Adsorption has been discovered to be one of the most effective methods for the elimination of these toxic metals from aqueous solution as a result of its high efficiency, cheap maintenance and very easy to apply. The most popularly used adsorbent is activated carbon due to its surface area and high adsorption capacity but it has the disadvantage of high cost, and this limits its application to small scale industries and developing nations. As a result, a search for cheaper alternative adsorbents has become a major area of interest for researchers. A good number of researchers have made use of low cost adsorbent materials for heavy metals removal. Some of the adsorbents used include Clay, Lateritic materials, Biomass, Red mud, and Sawdust.6 The use of natural agricultural waste material for the elimination of heavy metals ion through biosorption technique is very effective and several natural biosorbents have been exploited by researchers.7
However, despite the abundance and consumption of cowpea (Vignaunguiculata) in Nigeria and most countries, there is lack of information on the use of its leaves (which is left after harvesting of the pods) for the biosorption of heavy metals from solution. The leftover after harvesting (leaves, stem and root) serve as a source of energy for burning purposes. In search for cheaper biomass materials for the adsorption of heavy metals from solution, this study exploits the potential of Vigna unguiculata leaves (VUL) a low cost biosorbent for heavy metal remediation. The effect of various factors such as contact time, adsorbent dose, metal concentration, pH and temperature were investigated.
Materials and Methods
Biosorbent Preparation
The VUL were obtained after harvesting of the cowpea from the plant. The leaves were removed, rinsed with de-ionized water to get rid of unwanted materials and possibly some heavy metals attached to the surface. Then dried under the sun for several days and subsequently oven dried at 500C for 2hrs. The dried leaves were then crushed, grinded and pulverized to powdery form, then passed through 100µm mesh sieve to the Vigna unguiculata leaf powders (VULP) which was used for the biosorption process.
Adsorbate Preparation
Analytically grade chemicals were used in this study without further purification. A laboratory solution of lead (II), Cadmium (II) and Arsenic (III) ions were prepared by dissolving suitable amounts of Cd(NO3)2, Pb(NO3)2 and As2O3 respectively in 50ml of de-ionized water in a beaker and stirred properly with a glass rod to ensure proper dissolution. Thereafter the 50ml solution was then placed in a 1 liter volumetric flask and made up to the meniscus mark with de-ionized water to obtain a stock solution of concentration 1000mg/L of the metal ions. Several lower concentrations of the metal ions which include 200, 400, 600, and 800mg/L were then prepared from the stock solution by serial dilution.
Biosorption Study
Batch biosorption procedure was applied to determine the effect of contact time, Initial metal ion concentration, adsorbent dose, temperature and pHas described: To determine the effect of pH, the metal ion solution of concentration 200mg/L was used and varied with different pH values of 2.0, 3.0, 4.0, 5.0, 6.0, 7.0 and 8.0 by the drop wise addition of 0.1M NaOH or 0.1M HNO3, checked by a pH meter. Several 100ml plastic bottles were purified by washing with detergent then rinsed with de-ionized water and dried. 0.1g of VULP was placed in different plastic bottles and 20ml of metal solution was added. The plastics were corked, agitated for 5 seconds and then left for a contact time of 180min at a room temperature of 300K. At the end of a given contact time of biosorption the solution was filtered into another empty plastic using watmann no.1 filter paper placed in a funnel. The filtrate was then taken to the Atomic absorption spectrophotometer (AAS) (Buck scientific model 210VGP) to determine the concentration of metal ions remaining in solution.
To determine the influence of initial metal ion concentration several solutions of metal ions of concentrations 1000, 800, 600, 400, and 200mg/L were used. The pH of all the solutions was maintained at a constant pH of 6.0. 0.1g of VULP was in several 100ml plastic bottles after which 20ml of each solution was added, the plastics were corked then agitated for 5seconds and left to stand for 180min at a room temperature of 300K. The solutions were then filtered and taken to the AAS for residual metal concentration.
To determine the effect of biosorbent dosage, different weights of VULP of 0.1, 0.2, 0.3, 0.4 and 0.5g were weighed and placed in five 100ml plastic bottles. 20ml of the metal ion solution of concentration 200mg/L of pH 6.0 was added to the bottles, then corked agitated for 5 seconds and left to stand at 180min at a room temperature of 300K. The solutions were then filtered and the filtrate taken to the AAS to determine the concentration of metal ions remaining after biosorption.
To determine the effect of contact time, 0.1g of VULP was placed in eight 100ml plastic bottles. 20ml of metal ions solution of concentration 200mg/L of pH 6.0 was added to the bottles, agitated for 5 seconds after been corked and left to stand at different contact times of 10, 20, 30, 40, 50, 60, 90 and 120min. At the end of the given contact time for each experiment, the solutions were filtered and the concentration of metal ions remaining in the filtrated was determined from the AAS.
Determination of the effect of temperature on biosorption enabled the calculation of thermodynamic parameters. This was performed by varying the temperature of solution at which biosorption was conducted from 300, 313 and 323K with the help of a thermo-stated water bath. 0.1g of VULP as contacted with 200mg/L solution of the adsorbate at pH 6.0 and a contact time of 120min. The solution were filtered at the end of the given time and analyzed with the AAS.
Calculation of Biosorptive Removal and Biosorption Capacity
The percentage biosorption and the biosorption capacity of VULP for lead, cadmium and arsenic ions from solution were calculated from the given equations:
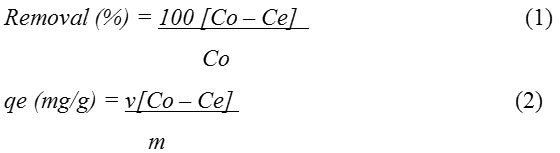
Where qe (mg/g) is the biosorption capacity, Co (mg/L) is the initial metal ion concentration in solution, Ce (mg/L) is the metal ion concentration remaining in solution at equilibrium, v (litres) represents the volume of solution used for the biosorption and m (g) is the mass of the biosorbent utilized.
Biosorptive isotherm analysis
The biosorption isotherm analysis of metal ions on VULP was studied by the application of the Langmuir, Freundlich, Scatchard and Flory-Huggins equilibrium models.8 The Langmuir isotherm is expressed in its linear form as:
Ce/qe 1/qLKL + Ce/qL (3)
Where qL (mg/g) is the monolayer biosorption capacity and KL (L/mg) is the Langmuir adsorption constant. Information on the nature of biosorption can be obtained from a dimensionless constant separation factor (RL):
RL = 1/[1 + KLCi] (4)
The RL identifies the biosorption to be favorable (0 < RL < 1), irreversible (RL = 0), linear (RL = 1) and unfavorable (RL> 1).
The Freundlich model is represented in its linear form as:
logqe = logKF + [1/n]logCe (5)
Where n and KF (L/g) represents the biosorption capacity and intensity, respectively and a favourable biosorption is indicated by values of n between 1 and 10.
The Scatchard isotherm provided further information on the homogenous or heterogonous form of the biosorbent and is expressed as:
qe/Ce = qSb – qeb (6)
Where qS (mg/g) and b (L/mg) are the Scatchard model biosorption parameters.
The Flory-Huggins model provides information on the degree of surface coverage of the biosorbent and is expressed linearly as:
log(θ/Ci) = logKFH+ nFHlog(1 – θ) (7)
Where θ = (1 – Ce/Ci) is represents the extent of surface coverage, KFH (L/g) and nFH are the Flory-Huggins model constant and model exponent, respectively.
Kinetic Model Analysis
Biosorptive kinetic modeling of metal ions unto VULP was performed by applying the Bangham, Pseudo-second-order (PSO), Elovich, and Pseudo-first-order (PFO) rate equations.9
The PFO kinetic model is expressed in its linear form as:
log (qe –qt) = log qe – (Kit/2.303) (8)
Where qt (mg/g) is the biosorption capacity at time t (min) and Ki (min-1) is the PFO rate constant of biosorption.
The PSO kinetic model is expressed in its linear form as:
t/qt = 1/K2qe2 + t/qe (9)
Where K2 (g/mg/min) represents the PSO rate constant of biosorption and h = K2qe2 is the initial sorption rate (mg/g/min).
The Elovich equation was applied in its linear form as:
qt = [1/β]ln(αβ) + [1/β]lnt (10)
Where β (g/mg) is a constant corresponding to the activation energy and surface coverage for chemisorptions, α (mg/g min) is the initial biosorption rate.
The Banghams kinetic model equation was applied in its linear form, expressed as:
loglog [Ci/(Ci – qtm)] = log (KOm/2.303V) + aBlog(t) (11)
Where V (ml) represents the volume of solution, KO (g) and aB (< 1) are Banghams constants.
Thermodynamic Analysis
Thermodynamic biosorption parameters; Enthalpy change (∆H˚), Gibbs free energy change (∆G˚), and Entropy change (∆S˚) were evaluated to provide information on the feasibility,spontaneity and heat change of the biosorption using the equations [8]:
∆G˚ = – RTlnKc (12)
lnKc = – (∆H˚/RT) + (∆S˚/R) (13)
Where Kc is the distribution coefficient, R (8.314 J/mol K) represents the ideal gas constant and T (K) is the absolute temperature of biosorption.
Results and Discussion
Influence of Initial pH and Metal Ion Concentration
Initial pH of solution is one of the most important factors affecting biosorption of metal ions on a biosorbent. This is because it affects the degree of ionization of metal ions and surface charge of the biosorbent.10 Figure 1 shows the influence of initial pH of solution against the biosorption of Pb(II), Cd(II) and As(III) unto VULP. A rise in biosorption of all metal ions with rise in solution pH was obtained. The biosorption of the three metals increased steadily up to pH 6.0 after which it became stable with further increase in pH. It should be noted that at higher pH values greater than 6.0 there could be precipitation of the insoluble hydroxide forms of the metal ions in solution. Therefore in this study to avoid metal precipitation associated with higher pH value and achieve optimum biosorption, pH value of 6.0 was chosen for all subsequent experiment. The low biosorption of all metals at low pH values is because at reduced pH more H+ ions are present in solution and competes with the metals for the active sites on VULP. This subsequently resulted in lower biosorption potential. As the pH increased, the number of hydrogen ions in solution decreases thereby reducing the competition with the metals for the active sites on VULP.2 This resulted in more sites available for metal ions binding leading to a higher biosorption with increase in pH. Furthermore, comparing the biosorption of the three metal ions, As (III) recorded the highest, followed by Cd (II) and then the least was Pb (II) ions. The differences in the amount adsorbed might be due to differences in the ionic radii of the metals, where metals with smaller ionic radii can easily diffuse to the surface of the adsorbent for higher adsorption than metals with larger ionic radii.5 The ionic radii of the metals are as follow As (III) (0.58Å), Cd(II) (0.97Å) and Pb(II) (1.20Å). This implies that As(III) was adsorbed more due to its smaller ionic radii while Pb(II) has the least adsorption due to its larger ionic radii. The electro-negativity of metal ions has also been reported to account for the differences in the level of metal ions adsorbed by an adsorbent.3
For efficient removal of metal ions from solution, initial metal ion concentration is an important factor for the biosorption of metal ions on biosorbents.Figure 2 shows the effect of initial metal ion concentration on the biosorption of Pb(II), Cd(II) and As(III) ions on VULP. Increased initial concentration of the three metal ions in solution with reduced percentage removal was obtained.The reduction in biosorption is as a result of constant number of active sites of all biosorbents at increased concentrations and saturation of the active sites.6 The saturation of the active sites at increased concentration subsequently gives rise to decrease in percentage biosorption of metal ions. The metals concentration of 200mg/L was then utilized in this study due to the highest adsorption obtained at this concentration. Similar results have been reported. 2,5,6,11,12
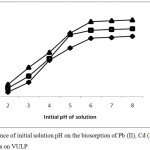 |
Figure 1: Influence of initial solution pH on the biosorption of Pb (II), Cd (II) and As (III) ions on VULP.
|
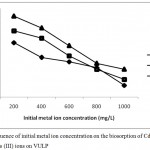 |
Figure 2: Influence of initial metal ion concentration on the biosorption of Cd (II), Pb (II) and As (III) ions on VULP.
|
Influence of Contact time and Biosorbent Dosage
The biosorbent dose is a very important factor which significantly affects the biosorption of metal ions from aqua media. Figure 3 shows the influence of biosorbent dose on the percentage biosorption of Pb(II), As(III) andCd(II) unto VULP. Increase in the dosage of VULP biomass from 0.1 to 0.5g gave rise to a corresponding increase in the percentage biosorption for the three metal ions. This is as a result of rise in the surface area provided by increasing biosorbent dosage and the presence of more active binding sites on the surface of the biosorbent.3 However, the use of excess amount of adsorbate in column experiment can determine the maximum biosorption capacity of an adsorbent.13
The time taken for equilibrium biosorption is important as an efficient biosorbent should not only have a high biosorption capacity but also a fast rate of removal. The influence of contact time on the percentage biosorption of Pb(II), As(III)and Cd(II) from solution on VULP is shown in Fig.4. Initially the rate at which the metal ions in the solution were removed was rapid and then there was a gradual decline in the rate until an equilibrium time beyond which there was no significant increase in the rate of biosorption. The fast biosorption at the initial stages is as a result of the presence of vacant and sufficient active sites on VULP which becomes used up with time, gets saturated thereby attaining equilibrium.14 Equilibrium was established around 20min for As(III), 40min for Cd(II) and 50min for Pb(II) ions. The faster rate of biosorption of As(III) is due to the smaller ionic radii which makes easy to diffuse faster to the surface of VULP when compared to the other metals ions. The same also applies for the faster rate of Cd(II) adsorption than Pb(II) ion on VULP biosorption potential. 180 mins was the contact time chosen in this study to enable equilibrium biosorptive removal was attained for the three metal ions. Similar observations have been documented by other researchers.2,5,6,7
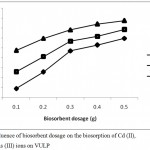 |
Figure 3: Influence of biosorbent dosage on the biosorption of Cd (II), Pb (II) and As (III) ions on VULP.
|
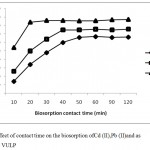 |
Figure 4: Effect of contact time on the biosorption ofCd (II),Pb (II)and as (III) ions on VULP.
|
Biosorptive Isotherm Modeling
Biosorption isotherm provides valuable information on the relationship or affinity between the biosorbent and adsorbate at constant temperature and at equilibrium. They give useful data interpretation on sorption mechanism and surface properties. The Flory-Huggins, Freundlich Langmuir and scatchard isotherm models parameters obtained from the biosorption of Cd (II), Pb (II) and As (III) from solution on VULP are presented in Table 1. It was elucidated from the R2 values (> 0.980) presented by the Langmuir model represented a very good fit of the model in the biosorption of the three metal ions on VULP. Furthermore the values of RL for the three metal ions biosorbed on VULP were in the range 0.04-0.28 which showed a favorable biosorption process. This favorability simply indicates the suitability of VULP as an efficient biosorbent for removal of Cd (II), Pb (II) and As (III) from contaminated wastewaters. The implication of the good fit of the Langmuir model to the biosorption process is that the surface of VULP is homogenous in nature and involves only a monolayer metal ion biosorption on the surface. The maximum monolayer biosorption capacity qLof the Langmuir isotherm showed the trend As (III) > Cd (II) >Pb (II) for metals biosorption which corroborated that obtain from the effect of observable parameters discussed previously. The R2 obtained from the Freundlich model on the other hand were lower than those of the Freundlich model which implies the inapplicability of this model in the description of the biosorption process. However, the n values were all between 1 and 10 which supports the RL value of the Langmuir model on the favourable biosorption process between VULP and Pb, Cd and As ions. Although the R2 values of the Flory-Huggins model were high, however they were lower than those of the Langmuir models and the assumptions of this model were not considered in this biosorption process. To confirm if the biosorbent contains only one type of active site (homogenous surface) the scatchard model was useful. If a straight line is obtained from the scatchard plot of qe/Ce against qe, then the biosorbent surface is Homogenous (Langmuir model fit), but a deviation from linearity indicates a heterogeneous biosorbent surface (Freundlich model fit).8 The R2 values of the scatchard model obtained for the three metal ions were very high (>0.99) which supports clearly the good fit of the Langmuir model indicating a homogenous surface of VULP biosorbent for monolayer sorption of the metal ions.
Table 1: Biosorptive isotherm model constants of metal ions removal by VULP.
| Isotherm/Adsorbent | Pb (II) | Cd (II) | As (III) |
| Langmuir | |||
| qL (mg/g) | 105 | 109.1 | 119.3 |
| KL (L/mg) | 0.013 | 0.017 | 0.024 |
| R2 | 0.996 | 0.991 | 0.982 |
| Freundlich | |||
| KF | 6.31 | 8.34 | 9.76 |
| N | 3.143 | 3.418 | 3.672 |
| R2 | 0.973 | 0.962 | 0.921 |
| Flory-Huggins | |||
| KFH (L/g) | 0.02 | 0.05 | 0.08 |
| nFH | 1.23 | 1.64 | i.87 |
| R2 | 0.942 | 0.921 | 0.903 |
| Scatchard | |||
| qs (mg/g) | 70.53 | 67.29 | 63.62 |
| b (L/mg) | 0 | 0 | 0 |
| R2 | 0.995 | 0.991 | 0.992 |
Kinetic and Thermodynamic Analysis of Biosorption
The kinetic model parameters of PFO, PSO, Elovich and Banghams rate equations obtained for the biosorption of Cd (II), Pb (II) and As (III) on VULP are presented in Table 2. The R2 values obtained from the PFO model for the three metal ions were low (<0.9) which indicated that the PFO model was not suitable in the description of the biosorption process on VULP. On the other hand the R2 values obtained for the PSO model were very high (>0.98) for the three metal ions. This indicated a good fit of the PSO model to the biosorption data. The implication of the good fit of the PSO model suggests internal diffusion mechanism and considers that biosorption is of a chemical nature.15 Several researchers have found the PSO to be more suitable in the description of kinetic mechanism of biosorption.3,4,6,11 The Elovich chemisorptions equation on the other hand also presented a good fit (R2> 0.9) but was lower than that of the PSO model. However the good fit of the Elovich chemisorption data to the biosorption supports the existence of chemisorptions mechanism and suggest also that physisorption is not the rate controlling mechanism. The R2 (>0.91) presented by the Bangham model for the biosorption process of the three metal ions on VULP was also very high (implied linear graph) which showed that the diffusion of Cd (II), Pb (II) and As (III) into the pores of VULP was significant in the diffusion mechanism of the metal ions on the biosorbent.16
Table 2: Kinetic model constants for the biosorption of metal ions on VULP.
| Kinetic/Adsorbent | Pb (II) | Cd (II) | As (III) |
| Pseudo-first-order | |||
| qecal(mg/g) | 42.14 | 34.26 | 48.13 |
| KI (min-I) | 0.001 | 0.007 | 0.011 |
| R2 | 0.863 | 0.814 | 0.742 |
| Pseudo-second-order | |||
| qecal (mg/g) | 46.4 | 50.32 | 53.91 |
| h(mg/g min) | 1.107 | 1.838 | 2.825 |
| K2(g/mg min) | 5.14 × 10-4 | 7.26 × 10-5 | 9.72 × 10-4 |
| R2 | 0.994 | 0.996 | 0.987 |
| Elovich | |||
| α (mg/gmin) | 0.514 | 0.627 | 5.1160.711 |
| β (g/min) | 0.042 | 0.061 | 0.083 |
| R2 | 0.903 | 0.933 | 0.927 |
| Banghams | |||
| aB | 0.187 | 0.199 | 0.214 |
| Ko (g) | 2.03 | 2.87 | 3.02 |
| R2 | 0.956 | 0.972 | 0.913 |
Furthermore, the thermodynamic parameters obtained from the biosorption of the three metal ions on VULP are shown in Table 3. Negative ∆H˚ obtained for all metal ions biosorbed indicated an exothermic biosorption process suggesting lower temperature favors the removal process. This result agrees with the good fit of the experimental data to the Langmuir isotherm model. Also, negative values obtained indicated a decrease in randomness at the solid solution interface. Negative values of ∆G˚ obtained at all temperatures for the three metal ions on VULP showed a spontaneous and feasible biosorption process and corroborated the favourable biosorption deduced from the Langmuir and Freundlich constants RL and n respectively. To obtain clarity on the classification of biosorption, if ∆H˚ value lie in the range 2.1 – 20.9 KJ/mol it indicates a physisorption process and between 80 – 200 KJ/mol indicates chemisorptions.5 The ∆H˚ values obtained for all the three metal ions were all greater than 20 but less than 80 KJ/mol indicating a physicochemical process rather than a solely physical or chemical biosorption.17 However even though the ∆H˚ values obtained were not up to the chemisorptions range, they were clearly greater than physisorption range which rules out physisorption as the dominant mechanism of the process. The good fit of the data to the Langmuir model and PSO model suggested chemisorptions process must have been the dominant mechanism of biosorption of Cd (II), Pb (II) and As (III) ions on VULP biomass. Strong desorbing agents might be required in desorption of the metal ions from the metal loaded VULP biomass.
Table 3: Thermodynamic parameters for the biosorption of heavy metals on VULP.
| Metal ion | Temp (K) | ∆G0 (kJ/mol) | ∆H0 (kJ/mol) | ∆S0 (J/molK) |
| 300 | -3.23 | |||
| Pb (II) | 313 | -2.71 | -30.26 | -42.41 |
| 323 | -2.26 | |||
| 300 | -5.31 | |||
| Cd (II) | 313 | -4.62 | -34.81 | -63.81 |
| 323 | -3.47 | |||
| 300 | -7.01 | |||
| As (III) | 313 | -5.98 | -39.36 | -90.44 |
| 323 | -4.26 |
Conclusion
The potential of VULP for the biosorption of Pb(II), Cd (II) and As (III) from solution was studied by batch methodology. The Langmuir model gave the best fit for the biosorpion of the three metal ions from solution compared to the Freundlich and Flory Huggins model. The Scatchard plot verified the existence of a homogenous surface of VULP. The Pseudo second order model gave best fit to the biosorption process than the pseudo-first order, Elovich and Banghams kinetic models. Biosorption thermodynamics showed a spontaneous, feasible and exothermic removal of the three metal ions from solution on VULP. The experimental data obtained in this study clearly showed the effectiveness and efficient potential of Vigna unguiculata seed powders as new cheap biosorbent for removal of Pb (II), Cd (II) and As (III) from contaminated media. The biomass was found to be effective under experimental conditions of pH, metal ion concentration and biosorbent dosage.
References
- Kobya M.,Demirabis E., Senturk E., Ice M. Bioresource Technology. 2005;96:1518-1521.
CrossRef - Dawodu F. A., Akpomie G. K.,Ogbu I. C. Inter. J. Multidiscip. Sci.Eng. 2012;3:21-26.
- Barka N.,Abdennouri M.,Makhfouk M. E., Qouezal S. J. Environ. Chem. Eng. 2013;1:144–149.
CrossRef - Mandal S.,Sahu M. K., Patel R. K. Water Resour. Ind. 2013;4:51-67.
CrossRef - Dawodu F. A.,Akpomie K. G. J. Mater. Res. Technol. 2014;3:129-141.
CrossRef - Das B., Mondal N. K. Uni. J. Environ. Res. Technol. 2011;1:515-530.
- Eluke L. O., Akpomie K. G., Chukwuemeka-Okorie H. O., Ajiwe V. I. Leonardo J. Sci. 2017;16:57-79.
- Dawodu M. O., Akpomie K. G. Alexand. Eng. J. 2016;55:3211-3218.
- Akpomie K. G., Onoabedje E. A., Alumona T. N., Alum O. L., Okagu O. D.,Ezeofor C. C. J. Environ. Sci. Manage. 2017;20:17-27.
- Imamoglu M., Tekir O. Desalination. 2008;228:108-113.
CrossRef - Karthikeyan G.,Anbalagan K., Muthulakshmi A. N. J. Chem. Sci. 2010;116:119-127.
CrossRef - Meitei M. D., Prasad M. N. J. Environ. Chem. Eng. 2013;1:200–207.
CrossRef - Zafar M. N.,Nadeem R., Hanif M. A. J. Hazard.Mater. 2006;143:478-485.
CrossRef - Gupta V. K., Jain C. K., Imran A., Sharma M., Saini V. K. Water Resources. 2003;37:4038-4044.
- Chiou M. S., Li H. Y. Chemosphere. 2003;50:36-42.
CrossRef - ManeV. S., Mall I. D., Srivastava V. C. J. Environ. Manage. 2007;84:390-400
CrossRef - Liang S., Guo X., Feng N., Tian Q. J. Hazard. Mater. 2010;174:756-762.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





