Manuscript accepted on : 16 February 2018
Published online on: --
Biological Function (S) and Application (S) of Pectin and Pectin Degrading Enzymes
Maharishi University of Information Technology (Established vide Uttar Pradesh Act No.31of 2001) Sitapur Road (IIM Bypass, Bhitauli Tiraha, P.O-Maharishi Vidya Mandir, Lucknow-226013 (UP), India.
Corresponding Author E-mail: parasar.pooja@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2611
ABSTRACT: Pectin is an integral part of plant cell wall and since centuries pectin extracted from plants is widely used in food and fruit juice processing. Moreover, in last half century, the applications have also invaded into many bio-processing applications such as pharmaceutical, bioenergy, textile, paper and tea processing. In these growing industries, the use of pectinases has grown with a significant amount i.e. approximately 10 % of total global enzyme market comes from pectinases. Herein comprehensive analyses of information related to structure and function of pectin in plant cell wall as well as structural classes of pectins have been discussed. The major function of pectin is in cementing the cellulose and hemicelluloses network, cell-cell adhesion and plant defence. Keeping the wide use of pectin in food industry and growing need of environment friendly technology for pectin extraction has accelerated the demand of pectin degrading enzymes (PDEs). PDEs are from three enzyme classes: carbohydrate esterases from CE8 and CE12 family, glycoside hydrolases from GH28 family and lyases from PL1, 2, 3, 9 and 10. We have reviewed the literature related to abundance and structure-function of these abovementioned enzymes from bacteria. From the current available literature, we found very limited information is present about thermostable PDEs. Hence, in future it could be a topic of study to gain the insight about structure-function of enzymes together with the expanded role of thermostable enzymes in development of bioprocesses based on these enzymes.
KEYWORDS: Pectin; Pectin Degrading Enzymes (PDEs); Glycoside Hydrolases; Pectate Lyases
Download this article as:| Copy the following to cite this article: Chandrayan P. Biological Function (S) and Application (S) of Pectin and Pectin Degrading Enzymes. Biosci Biotech Res Asia 2018;15(1). |
| Copy the following to cite this URL: Chandrayan P. Biological Function (S) and Application (S) of Pectin and Pectin Degrading Enzymes. Biosci Biotech Res Asia 2018;15(1). Available from: https://www.biotech-asia.org/?p=29369 |
Introduction
Pectin is abundant in middle lamella and primary cell wall of various terrestrial plants and tissue types like fruits and leaves (Rose 2003; Lerouxel et al. 2006; Scheller et al. 2007).The amount of total pectin is highest in dicotyledonous plants and nongraminaceous (nongrass) monocots such as apples, oranges, cherries, sunflowers, grapes, bananas and tomatoes. It is also present in grasseous plants like maize, wheat, sorghum, barley etc but total amount is lower than dicots. Primary cell wall of dicot plants contain 35% pectin while grasses have approximately 2-10% pectin (Cosgrove 2005; Wolf and Greiner 2012; Atmodjo et al. 2013). Pectins extracted from orange and apple is currently major source and in extraction role of pectin degrading enzymes are central. In this review, current status of literature and future trends has been described in detail.
Functional Role of Pectin in Plant Cell Wall
As a part of the cell wall, pectin plays major role in providing structural support to plant (Palin and Geitmann 2012; Wolf and Greiner 2012; Atmodjo et al. 2013; Daher and Braybrook 2015). Primary cell wall is very rich in a cross-linked network of cellulose and hemicelluloses but role of pectin is pivotal in cementing this network. The structural studies present a model in which the role of pectin is modelled as a matrix into which the cellulose and hemicelluloses fibrils are embedded and glued together as a structural unit. A representative depiction of a typical primary cell wall is shown in figure 1. From the proposed model, the special role of pectin is very significant and mainly lies in its intrinsic capability to form a coagulated gel. Indeed, the name pectin is derived from the greek word pēktos that stand for congealed or coagulated. The middle lamella, as shown in figure 1, contain abundant of pectins and these pectins play a necessary role in the formation of cell-cell adhesion among various plant tissues (Willats et al. 2001; Malinovsky et al. 2014). Here, the role of calcium in formation of adhesive interaction is well documented and experimentally confirmed too. Calcium cross-links the pectin component of two middle lamellas through the negative charge present in the monomeric unit galacturonic acid. Other factors responsible for cell-cell adhesion and interaction are the side chain sugars attached to backbone formed by polygalacturonan (Scheller et al. 2007; Palin and Geitmann 2012). Among these side-chains, some sugars some are very common hexose and pentose sugars such as arabinose, galactose and xylose but many of them are peculiar and rare sugars like apiose and aceric acid- a branched chain sugar, 3-deoxy-manno-2-octulosonic acid (KDO) – an eight carbon containing sugar and 3-deoxy-lyxo-2-heptulosaric acid (DHA)-a seven carbon containing sugar. In literature, several plant mutants lacking these rare sugars have been reported and in these mutants loss of cell-cell adhesion have been noticed because of the altered composition of the pectin in middle lamella (Cosgrove 2005; Mohnen 2008; Daher and Braybrook 2015). In addition, pectin has also an effect on porosity, surface charge, pH and ion balance in the cell wall.
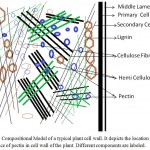 |
Figure 1: Compositional Model of a typical plant cell wall. It depicts the location and importance of pectin in cell wall of the plant. Different components are labeled.
|
Furthermore, pectin oligosaccharides are known to activate the plant defence mechanism by accumulation of phytoalexin, which displays antimicrobial activity and also stimulate the lignification in plant tissues (Malinovsky et al. 2014). Phytopathogenic fungi secrete pectin degrading enzymes (PDEs) to gain the entry into the plant tissues. Subsequently, PDEs produce oligogalacturonide (OGA) fragments from the pectin backbone, which act as potent defence response elicitors (Collmer and Bateman 1982; Gonzalez-Candelas and Kolattukudy 1992; Lara-Márquez et al. 2011; Xie et al. 2012; Tsuyumu et al. 2014).
Altogether, pectin is mainly a structural polysaccharide present in the plant cell wall and has major function in holding the overall structure of the plant together with cellulose and hemicelluloses network (Lerouxel et al. 2006; Malinovsky et al. 2014; Tsuyumu et al. 2014). Moreover, it also plays a significant role in plant-pathogen interaction, where degradation products of pectin have been shown as a major molecule to activate the pathogen protection machinery present in plants.
The Structural Classes of Pectin
Pectin is a hetero-polymer and perhaps the most complex macromolecule present in the plant cell wall, having 17 different types of monosaccharide as well as 20 different types of linkages to assemble these sugars (Rose 2003; Atmodjo et al. 2013). Nevertheless, approximately 65% of cell wall pectin is a homopolymer of α-D-galacturonic acid (figure 2). Hence, α-D-galacturonic acid is the major monosaccharide found in pectin. However, studies of sugar compositions and its linkages have led to the view that there are three major type of pectic polysaccharides are present: (1) Homogalacturonan (HG), (2) Rhamnogalacturonan I (RG-I) and (3) Rhamnogalacturonan II (RG-II) (Mohnen 2008). In figure 2, a simplified representation of all three groups of pectin is shown (Scheller et al. 2007; Harholt et al. 2010).
Homogalacturonan (HG):
HG is a homoplymer of α-D-galacturonic acid. The glycosidic linkage between the backbone is a α-1, 4 configuration. The monomeric unit is a sugar acid, an oxidized form of D-galactose. A pyransose ring form of the α-D-galacturonic acid is shown in figure 2. Based on this, it has an aldehyde group at C1 and a carboxylic acid group at C6. The carboxylic acid at position C6 occurs in the form of methyl ester too (As shown in figure 2). Degree of esterification (DE) determines the physical properties of the pectin and based on esterification, industrial quality of pectins are classified into two broad categories: HM (high methoxy) and LM (low methoxy) pectin as described by IPPA- international pectin producers association). HM pectin has greater than 50% DE whereas LM pectin has less than 50% DE. HM and LM pectins form the gel by different mechanism because of differential amount of esterification (Leroux et al. 2003; Caffall and Mohnen 2009). HM pectin requires a minimum amount of soluble solids such as sugars and a narrow pH range, around 3.0, in order to form gels. Also, these gels are thermally reversible. In general, HM-pectins are hot water soluble and often contain a dispersion agent such as dextrose to prevent lumping. In comparison, LM-pectins yield gels independent of sugar content and the gel formation is not sensitive to pH. LM-pectins need presence of a controlled amount of calcium or other divalent cations for gelation. Based on these properties they have their unique set of industrial applications (May 1990; Dominiak et al. 2014b). Like, esterification, also in the some cases the amidation of carboxyl group at C6 position occurs but the amount of amidation is too low to influence the gelling properties (Scheller et al. 2007; Caffall and Mohnen 2009). Similarly acetylation of the oxygen atom of position O2 and O3 has been also seen in some natural pectin. Acetylation of α-D-galacturonic acid changes the distinct gelling properties of pectin in negative manner.
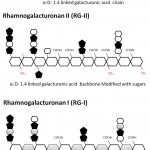 |
Figure 2: Figure 3 A schematic diagram of the three major types of pectins– HG (Homogalacturonan), RG-I (Rhamnogalacturonan I), RG-II (Rhamnogalacturonan II).
|
Rhamnogalacturonan II (RG-II)
RG-II amount to approximately 10% of the total pectin and is the most composite but structurally the most conserved group of pectin found in nature. It has similar backbone like HG but there are four conserved side chains A, B, C and D are invariably present. Side chain A and B are an octasaccharide and nonasachharides respectively linked to HG backbone via β-D-apiosyl (Apif) at O2 position. Side chain C and D are chemically a disaccharide and is linked to O3 position of polygalacturonate backbone. RG-II has 12 different types of sugars in more than 20 linkages. The conserved nature of RG-II pectins suggests a key function in plant growth and development (Srivastava and Malviya 2011; Atmodjo et al. 2013). Expectedly, it has been reported that a minor modification of these conserved sugar and linkage pattern may result into a deleterious effects on plant growth including dwarfism. Also, in the network structure of the cell wall, generally, they are cross-linked via borate in the plant tissue (Palin and Geitmann 2012; Atmodjo et al. 2013).
Rhamnogalacturonan I (RG-I)
It comprises 20-35% of total pectin and is significantly unrelated in the backbone structure. HG and RG-II has a similar backbone of α-1,4 linked D-galacturonic acid but here the backbone is a repeating unit of a disaccharide: -[4)-α-D-galacturonic acid-(1,2)-α-L-rhamnose-(1]. Also, in HG the most abundant modification is esterification at C6 position whereas in RG-I, the acetylation at O2 and O3 position of α-D-galacturonic acid is more prevalent (Scheller et al. 2007; Caffall and Mohnen 2009).
Very clearly, the HG is most abundant pectin but on the whole, in a typical plant cell wall, how these three types of pectin are arranged and cross-linked to each other is not well understood (Willats et al. 2001; Mohnen 2008; Atmodjo et al. 2013). Nonetheless, the experimental reports are indicative of covalent linkages between them. One of the roadblocks to gain further understanding about the structural association of these three type of pectin lies in the isolation of intact native pectin from the plant cell wall. Generally, chemicals and enzymes used in isolation and enrichment of pectin fraction destroy the structural linkages present in the native pectin (Mohnen 2008; Caffall and Mohnen 2009). Therefore, in future, more advanced techniques of cell imaging will be indispensible to gain insight into function of diverse classes of pectin in cell wall.
Applications of Extracted Pectins
Pectin is common in daily used cereals and fruits so, the intake of pectin in human diet is obvious and in the digestive system it is metabolized by the gut-microbes. Hence, intake of pectin in human diet has prebiotic effect as well as it is an excellent source of soluble dietary fibre (Thakur et al. 1997; Sriamornsak 2003; Munarin et al. 2012; Garg et al. 2016). There are several reports linked to consumption of pectin as a food material. One of the study shows that the chamomile extract from vegetable pectin reduce the diarrhoea related symptoms (Iglesias and Lozano 2004). Another study revealed that the pectic oligosaccharides found in carrot soup are very effective in minimizing the gastro-intestinal infections (Srivastava and Malviya 2011; Munarin et al. 2012). Furthermore, the pectin intake lowers the blood cholesterol level and act as controlling agent of blood glucose level in diabetic patients (Srivastava and Malviya 2011; Munarin et al. 2012). In addition, from decades pectin is used in food products like Jam, yoghurt drinks, jelly and fruity milk drinks, predominantly as gelling, stabilizing or thickening agent (May 1990; Sriamornsak 2003; Sharma et al. 2006; Xiao and Anderson 2013; Latarullo et al. 2016). According to IPPA (International Pectin Producers Associations), pectins have following major applications: (1). Fruit applications: Jams, jellies, and desserts ;( 2). Fruit preparations used in bakery fillings and toppings ;( 3) Dairy industries: acidified drinks and protein drinks ;( 4) Confectionery and beverages; (5) Nutritional and health products and (6) Pharmaceutical and medical applications (BeMiller 1986; Thakur et al. 1997; Sriamornsak 2003; Sharma et al. 2006; Munarin et al. 2012; Tapre and Jain 2014; Garg et al. 2016).
Although structurally, pectins are of three major class but applications are more confined to homogalacturonan. Also, pectins in these abovementioned applications are extracted from plant sources, so US Food and Drug administration (FDA) has categorised them as GRAS (generally regarded as safe). As per recommended specifications for food grade pectin, the total galacturonic acid should be more than 65% calculated on the ash-free and dried bases. In some cases, the food grade pectin has amidation at C6 position and as per US FDA; the amount of amidation should not exceed 25% of total carboxyl groups. The data is freely available at the website: https://www.fda.gov/food/ingredientspackaginglabeling/gras/scogs/ucm260947.html and http://www.ippa.info/safety.html.
At present, the most common source of pectin is citrus and apple peels. The literature available on extraction methods are of two types: (1) direct boiling at low pH and (2) microwave based heating (Canteri-Schemin et al. 2005; Xu et al. 2014). Boiling is a conventional method and takes more than 2-4 hours to obtain the sufficient amount of pectin, later which is precipitated by organic solvents like methanol or ethanol. Although, it is a traditional method but there are few drawbacks like long duration and thermal degradation of the pectic substances. On the contrary, the microwave heating procedure does not take more than 15-20 minutes and produces the good quality pectin with a satisfactory yield (Canteri-Schemin et al. 2005; Liu et al. 2006; Ismail et al. 2012; Methacanon et al. 2014; Xu et al. 2014; Lefsih et al. 2017). Here, the both methods employed for extraction of pectin is based on use of nitric, hydrochloric or sulphuric acid. Therefore, the main disadvantage of this technology is generation of large volumes of acidic effluent, which need additional treatment before release into environment. Hence, enzymatic degradation to release the pectin from plant cell wall and thereby achieve sustainable, green production of pectin has become a topic of research in the last decade (Dominiak et al. 2014a; Wikiera et al. 2015). Consequently, now these days enzymatic processing of natural plant based material to obtain a sizeable amount of intact and functional pectin is more favoured.
Enzyme classes for Pectin Degrading Enzymes (PDEs)
Although there are three types of pectin present in plants but the major industrial and biotechnological applications are restricted to HG type (BeMiller 1986; Sharma et al. 2006). Thus, in this section for classification of PDEs, only those enzymes which are capable for hydrolyzing HG are included. For a better understanding the distinction between pectin and pectate lies in esterification. The former has esterification at C6 position whereas latter completely lack esterification. As discussed earlier based on extent of esterification there are two grades of pectin – low and high methoxy pectin.
According to type of linkages there are two categories of enzymes: de-esterifying and de-polymerising enzymes. The later enzymes are further classified into two classes based on the reaction mechanism – hydrolases and lyases (Dubey et al. 2010; Hugouvieux-Cotte-Pattat et al. 2014). Both enzymes break the α-1,4 linkages present in polygalacturonate but the former one use water for hydrolysis while the later one cleave the linkage, without water molecules, via β-elimination reaction (Tapre and Jain 2014; Garg et al. 2016; Yadav 2016). A simplified pictorial representation of the reaction, reaction products and position of bond cleavage is shown in figure 3 and enzyme classes are listed in table 1.1. Based on these, the pectin degrading enzymes (PDEs) are described below.
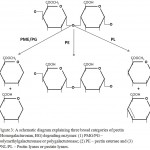 |
Figure 3: A schematic diagram explaining three broad categories of pectin (Homogalacturonan, HG) degrading enzymes: (1) PMG/PG – polymethylgalacturonase or polygalacturonase; (2) PE – pectin esterase and (3) PNL/PL – Pectin lyases or pectate lyases.
|
Pectin methylesterase (PME) (EC 3.1.1.11)
Perform the removal of the methoxyl groups from the C-6 carboxyl groups of galacturonate units by hydrolysis (Fig. 3). Subsequently, pectin acid, methanol and H+ from ionisation of carboxyl groups are formed. The release of H+ decrease the pH of the reaction media so, by the measurement of the pH of the solution after the reaction is an assay to determine the enzymatic activity (Yadav 2016).
There are many PME secreting microbes and these enzymes are listed and classified in a comprehensive database – CAZy (Carbohydrate Active enZymes) (Cantarel et al. 2009). In this CAZy database, PME belongs to carbohydrate esterase (CE) family and this group of enzyme is active on carbohydrate esters. Currently, there are seventeen classes (CE1 to CE16 and one unclassified family) of CEs and PMEs are grouped into CE8 family. This classification is based on evolutionary history of the genes encoding the PMEs and also it takes account of sequence similarities among the genes, reaction mechanisms and protein folds. So far, total 2794 gene sequences are reported in the CE8 family and a greater part of them are from bacteria and eukaryotes. From archaea, only 5 genes are reported till now. Among these only 47 proteins are studied in detail and from these, 8 proteins have three-dimensional structures deposited in Protein Data bank (PDB) (Laskowski 2001).
Here, our focus is on bacterial pectin degrading enzymes (PDEs) and among the 47 biochemical characterized enzymes; only three enzymes have been reported till date (as per the information available in public database) from bacterial domain (Marín-Rodríguez et al. 2002; Dubey et al. 2010; Hugouvieux-Cotte-Pattat et al. 2014) Interestingly, all the three enzymes are from family Enterobacteriaceae. These three enzymes are: (1) pectin methyl esterase (PmeA) of Dickeya chrysanthemi B374 /B364 (NCPPB898); (2) pectin methyl esterase A (PemA) of Dickeya dadantii 3937 and (3) pectin methyl esterase B (PemB) of Dickeya dadantii 3937 (Cantarel et al. 2009)
The recombinant purified PmeA displayed the maximum activity at pH 8.0 – 9.0 and at a temperature of 50°C. For the optimal activity, 50-100 mM monovalent cations or 5-10 mM divalent cations are essential and a specific effect of Ca2+ and Zn2+ on PmeA activity has been also seen (Plastow 1988; Shevchik and Hugouvieux-Cotte-Pattat 1997). The other two enzymes – PemA and PemB has also similar enzyme profile. Currently, available commercial preparation of PROZOMIX is from Streptomyces avermitilis MA-4680, but data related to biochemical studies is not available. From the specification sheet of the enzyme from the supplier, the optimum activity is at 30°C and pH 7.5.The three dimensional structure of PmeA and PemA confirms the presence of a typical β-helix found in PMEs (Plastow 1988).
Pectin acetylesterase (PAE) (EC 3.1.1.06)
Hydrolyze the pectin by removing acetyl groups from C2 and C3 hydroxyl groups of galacturonate units present in HG and RG-I and RG-II. The first pectin acetylesterase activity was reported in a fungus – Aspergillus niger (Kauppinen et al. 1995; Dinu et al. 2007; Martens-Uzunova and Schaap 2009). However, the studies of pectin acetylesterase are so far not enough to get a molecular detail of the enzyme activity and substrate specificity. PAEs cleave the acetyl ester linkage in the homogalacturonan region of pectin yielding pectic acid and acetate. Likewise PMEs, PAEs are classified into carbohydrate esterase (CE) families 12 and 13. There is a very little information about the members of CE13 family so; the knowledge of function and substrate specificity is from members of CE12 family.
At this time, there are 1216 gene sequences are available and more than 80% of these sequences are from bacteria and among the bacterial PAEs, only eight have been studied in terms of structures and functions. Pectin acetylesterase (YesY;BsPAE) of Bacillus subtilis and pectin acetylesterase (PaeY;Dda3937_03373) from Dickeya dadantii 3937 are the most studied PAEs and have the optimum pH and temperature in the range of 7.0 -9.0 and 40-50°C (Shevchik and Hugouvieux-Cotte-Pattat 2003; Grenier et al. 2006) . The enzyme profile data is from the synthetic substrate- pNP-acetate so; still the enzymatic activity with native substrate acetylated pectin has been not established. Surprisingly, there is no report of the confirmed three dimensional structure by X-ray crystallography or NMR (Nuclear Magnetic Resonance) for any characterized protein, yet the evolutionary history predicts a unique structure of (α / β/α)-sandwich unlike the β-helix of PMEs. In addition, CE12 family has also rhamnogalacturonan acetylesterases (RGAE) that carry out hydrolytic cleavage of acetyl groups from rhmnogalacturonan chain of RG-II. Commercially supplier PROZOMIX has PAE from Clostridium thermocellum which has the optimum temperature at 50°C and a pH of 6.5 and to the best of our knowledge there are no supplier of RGAE (Searle-van Leeuwen et al. 1992; Remoroza et al. 2014).
Conclusively, de-esterifying enzymes aim to remove the modifications like addition of methoxy and acetyl group. Although, we have not discussed the removal of sugar modifications of the polygalacturonate backbone but we need a set of specific hydrolases targeting the side chain sugars, as described earlier in the section “structural elements of pectin”. These specific and unique enzymes have been reviewed elsewhere in details (Atmodjo et al. 2013; Ndeh et al. 2017).
De-polymerising enzymes (Hydrolases)-Endopolygalacturonase (EC 3.2.1.15) and Exopolygalacturonase (EC 3.2.1.67)
These are known as polygalacturonases (PG) and catalyze the hydrolysis of α- 1,4-glycosidic linkages in polygalacturonic acid (PGA). Depending on the site of cleavage they are further divided as Endo-PG that cleaves the glycosidic bonds randomly and yields oligogalacturonate and Exo-PG that cleaves the bond from terminal and generally produces monogalacturonates. In addition, there is another class of Exo-PG, known as exo-poly-α-galacturonosidase (EC 3.2.1.82), that cleaves the penultimate bond rather than terminal bond from non-reducing end of PGA. Consequently, they produce digalacturonate as a product rather than monogalacturonate.
All three types of hydrolases are clustered into glycosyl hydrolase 28 family (GH28). So far, 21 bacterial proteins have been characterized as a member of GH28 and of these 12 are endo-PG, 7 are exo-PG and remaining 2 are exo-poly-α-galacturonosidase. Among these, there are four enzymes with their three-dimensional structures deposited in PDB. Structural information of these four proteins reveals a β-helix fold like PME. The overall fold is right-handed parallel β-helix, which is decorated with loops and secondary structure elements on one side (“top”), and a 26-residue N-terminal extension comprising of α-helix and a loop at the “bottom”. At the top side there is a conserved active site pocket, in which three aspartate residues are present at the bottom of the pocket. These aspartate residues are responsible for the hydrolysis of the glycosidic linkages present in the pectin and pectate and are known as active site residues. Based on the mechanism, these are inverting glycoside hydrolases where upon hydrolysis the anomeric configuration inverts in product. All three enzymes produce reducing sugars as a reaction product so, the enzyme assays to measure the Endo and Exo-PG activity is the standard assay for estimation of reducing sugars i.e 3,5-dinitrosalicylic acid (DNS) assay described by Miller (Miller 1959).
De-polymerising enzymes (Lyases)-Endopolygalacturonate lyase (EC 4.2.2.2) and Exopolygalacturonate lyase (EC 4.2.2.9)
The enzymes from EC 4.2.2.2/9 is an example of carbon-oxygen lyase. Besides, lyases have also the ability to break the carbon-carbon, carbon-nitrogen, carbon-sulfur, and carbon-halide and phosphorus-oxygen bond present in organic and inorganic compounds. The substrate utilized by pectate lyases (PL) is same like endo and exo-PG and cleaves the same carbon–oxygen bond of α-1, 4 glycosidic linkage between uronic acids but instead of yielding the saturated mono,di and oligo-galacturonic acids, PL produces the same product having a unsaturated C4-C5 bond. Also, these unsaturated oligo-galacturonates absorbs at 235nm, which is utilized for in vitro assay of pectate lyases. Thus far, an alkaline pH range 8.5-10.5 and need of a metal ion is the general trend exhibited by most of pectate lyases. Ca2+, Co2+, Mn2+, and Ni2+ are mostly used as a metal cofactor in the catalysis (Yadav 2016).
Like PGs, pectate lyases are also further divided as endo-PL that cleaves the glycosidic bonds randomly and exo-PL that cleaves the bond from terminal and generally produces unsaturated monogalacturonates. In addition, there is another class of exo-PG, known Oligo-D-galactosiduronate lyase (EC 4.2.2.6), that cleaves the penultimate bond rather than terminal bond from non-reducing end of PGA and yields di-galacturonates. In CAZy database, these enzymes are found in five different polysaccharide families: PL1, PL2, PL3, PL9 and PL10. Except PL1, which has more than 100 PLs from bacterial sources and these are well studied, remaining four pectate lyases have only a few well studied PLs from bacteria. Three-dimensional structures of many PLs have been determined and three different kinds of topologies are present in the five different families for the catalytic module: (1) a right-handed β-helix fold which is common with the families PL-1, PL-3, and PL-9; (2) an (α/α) 7 toroid in family PL2; and (3) (α/α) 3 toroid in family PL-10. β-helix fold is more abundant among different pectin degrading enzymes and is structurally very unique. As mentioned earlier, PMEs and PGs have also this typical β-helix fold (Yoder et al. 1993; Hurlbert and Preston 2000).
Despite variations in sequence similarities and protein folds of all five families, they still have a shared catalytic mechanism. On the contrary to glycoside hydrolase (PGs), polysaccharide lyase (PLs) breaks bond without involvement of water molecule and result in the formation of an unsaturated bond at the non-reducing end. The catalytic mechanism can be summarized into three steps: (1) abstraction of the C-5 proton on the sugar ring of a galacturonic acid or ester by a basic amino acid from the active site, (2) stabilization of the resulting anion by charge delocalization into the C-6 carbonyl group and (3) lytic cleavage of the O-4: C-4 bond, facilitated by proton donation from a acid residue from the active site to yield a hexenuronic acid and hexuronic acid (Garg et al. 2016; Praveen and Suneetha 2016).
Applications of Pectin Degrading Enzymes (PDEs)
In the previous section, we described the salient features of the enzymes linked to pectin degradation and now in this section, the applications of PDEs are outlined. The first commercial application of PDEs was for clarification of apple juice in 1930 by Kertesz. Since then, it has expanded for many applications as outlined in figure 4.
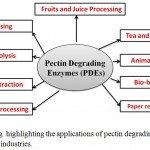 |
Figure 4: A drawing highlighting the applications of pectin degrading enzymes (PDEs) in different industries.
|
Fruits and vegetables processing
In fruit juice industries, a fruit juice preparations having low viscosity and high clarity is looked for trait. To achieve these traits, pectinases plays a key role by liquefaction (of fruit pulps by depectinization). For apple juice, pectinase treatment augments the transmittance by 143% and reduces the viscosity substantially (Praveen and Suneetha 2016).
Depectinization reactions are generally carried out in the pH range of 2.0 to 6.0 at 40-50°C for a variable time-period ranging from 5 minutes to 6 hours. For example, in citrus juice processing PDEs help in removal of cloudiness and in stabilization of juices. Moreover, cell wall degrading enzymes like cellulases and hemicellulases are also added to release the pectin from plant cell wall (Wikiera et al. 2015).
Application of enzymes is also related to fruit types. Soft fruits like banana, carrot and papaya results in gel like mess because of high content of pectin. In this particular case, pectinases is essential for improving the pressing characteristics. Indeed, addition of PDEs increases the overall juice-yield by 3-4 folds. In pineapple juice preparations, addition of pectinase with hemicellulases enhances the juice yield by 25% (Tapre and Jain 2014; Garg et al. 2016).
Pectinase treatment not only reduces the viscosity and enhances the clarity in juice preparations but also increase the release of polyphenols and phenolic components from fruit skin. These polyphenols has a proven role as an antioxidant that has many health benefits. Dragon fruit juice obtained by enzymatic treatment has 15% more phenolics. So, PDEs also improves nutritional value of fruit juices (Tapre and Jain 2014).
Wine making and processing
Fruit juices are the starting material for ethanolic fermentation. Pre-treatment of fruit juice enhance the ethanol yield, sensory characteristics of wine and stability of fermented products. In some case, pectinase treated wine has methanol because of the action of pectin/pectate methyl esterase (PME). Therefore, it is highly recommended that the pectinase enzyme mixture used in wine mixture must contain either no or a very restricted amount of PMEs (Hoondal et al. 2002).
Biomass hydrolysis and Biofuel
Pectin-rich biomass as sugar beet pulp, citrus waste, and apple pomace has shown promise as bioenergy feedstocks to produce bioethanol. Noticeably, these bioenergy feedstocks will require saccharification and fermentation methods that are optimized for the suite of sugars they contain and a tailored or engineered E. coli has been shown to use the pectin rich biomass for bioethanol production. Enzymatic hydrolysis of the feedstock is prerequisite for the pectin rich biomass refinery where the role of pectin degrading enzymes is central (Turner et al. 2007).
Although, in cellulosic biomass the pectin contributes a small amount but in a recent study it has been reported that the addition of pectate lyases in cellulase enzyme mixture reduces the enzyme loading by approximately 25-30%. Hence, the PDEs is also very effective in development of a cost-effective cellulase mixture for cellulosic ethanol (Xiao and Anderson 2013).
Extraction of vegetable oil
Vegetable oils of olive, sunflower, coconut, palm or canola are nowadays extracted with the use of alkaline pectolytic enzymes together with an enzyme concoction having cellulases, hemicellulases. Enzymatic treatment increase oil yield as well as boost the polyphenolic and vitamin E content. In summary enzyme based extraction of oil from oil seed plants is a green process and role of PDEs step up the yield and nutritional value of extracted oil (Kusuma and Sri Rami Reddy 2014).
Processing of textile material
Bio-scouring is a method for removal of non-cellulosic impurities from the fiber and cotton materials. Chemical methods are currently being replaced by eco-friendly enzymatic process. Alkaline pectinase has been found as the most appropriate enzyme for cotton scouring by many researchers but enzyme combinations like lipase alongside pectinase is more effective for bio-scouring of cotton fibre. Since last decade, the feasibility of enzymatic scouring to cotton, jute, coir, flax, hemp, ramie and banana has been attempted with success (Garg et al. 2016).
Tea and coffee Processing
The reduction in pectin content of tea leaves by pectinase treatment speed up the tea fermentation and reduces the foam forming property of instant tea powder and develops a characteristic aroma. Similarly, the removal of the mucilage layer of coffee bean improves the yield, reduction in the processing time, aroma and flavour of robusta coffee (Garg et al. 2016).
Processing of Animal Feed
Animal feeds are very rich in fibre and inclusion of cellulolytic, xylanolytic and pectinolytic enzymes enhance the food absorption in the rumen gut by lowering down the viscosity of the feed. This was quite evident in increase of animal weight upon inclusion of PDEs in the animal feed.
Biobleaching of the Kraft Pulp
The presence of pectins causes yellowness of paper in the sheet formation for the paper and pulp industry. On chemical bleaching the kraft pulp brightens the paper sheet by reducing the galacturonic acid content. The same reduction has been also achieved by enzyme and thus use of pectinase in the biobleaching is growing now these days. In fact, synergistic action of xylanase with pectinase in biobleaching of pulp has shown more promise in processing of kraft pulp (Kirk et al. 2002; Srivastava and Malviya 2011; Garg et al. 2016).
De-inking and Paper recycling
Deinking process is a key step for paper recycling and at present use of chemicals in the above process throws up a big environmental challenge for waste effluent treatment. Thus, the enzymatic de-inking by using a mixture of pectinases, hemicellulases, cellulases and lignolytic enzymes is more preferred. Enzymatic deinking modifies the bonds near the ink particle and remove ink from fibre surface. Subsequently, the released ink takes off by washing or floatation. Again, a combination of xylanase and pectinase has been successfully used for development of an eco-friendly process of the deinking of waste papers (Kirk et al. 2002; Srivastava and Malviya 2011; Kusuma and Sri Rami Reddy 2014; Li et al. 2014; Garg et al. 2016).
Conclusion and Future Outlook
Conclusively, PDEs have diverse applications centred on food and beverage industries as well as a potential application in area of bioenergy for biorefinery based on pectin rich feedstocks. A new research report by Global Market Insights has predicted the growth of industrial enzymes market is expected to cross USD 9.5 billion by 2024; and the major growth has been speculated for food & beverages and animal feed industry where pectin degrading enzymes alone have share of approximately 20-25% (Kumar et al. 2014; Liu et al. 2016). Thus, studies related to pectin degrading enzymes are a very active research area especially for thermostable pectin degrading enzymes. These thermostable enzymes will have major impact on fruit and vegetable processing, paper and pulp industry and in bioenergy. At present Commercially, Novozyme Inc is sole supplier of thermostable pectinase – Novozym 33095 which has a considerable stability up to 60°C and the optimum activity is in a range of 50-60°C (Sharma et al. 2013; Priya and Sashi 2014). So, novel and new thermostable pectinases are currently being sought to meet the growing demand.
However, in literature there are only a few reports of thermostable pectinase such as a PGase from Streptomyces sp. QG-11-3 is 60 °C (Beg et al. 2000), a pectinase from a hyperthermophilic bacterium Thermotoga maritima having temperature optima of 80°C (Kluskens et al. 2003; Pijning et al. 2009), a thermostable pectinase from Bacillus halodurans M29 having the similar temperature optima and a pectate lyase from Caldicellulosiruptor bescii with temperature optima in the range of 85-90°C. Hence, to meet the demand and better understanding of structure and functions of PDEs, the study of thermostable pectin degrading will be a hot topic for the research and many biotechnological appliications (Turner et al. 2007; Pedrolli et al. 2009; Kusuma and Sri Rami Reddy 2014; Su et al. 2015).
Acknowledgement
PC and AB thanks MUIT, Lucknow for providing the facility for carrying out the study.
Conflict of Interest
PC and AB confirm no conflict of interest in the work reported.
Financial Aid
Not any financial aid was given for the work.
Ethics of human and animal experimentation
The paper does not involve any human and animals for carrying out any experimental work.
References
- Atmodjo M.A, Hao Z, Mohnen D. Evolving Views of Pectin Biosynthesis. Annu Rev Plant Biol. 2013;64:747–779. doi: 10.1146/annurev-arplant-042811-105534
CrossRef - Beg Q.K, Bhushan B, Kapoor M, Hoondal G.S. Production and characterization of thermostable xylanase and pectinase from Streptomyces sp. QG-11-3. J. Ind Microbiol Biotechnol. 2000;24:396–402. doi: 10.1038/sj.jim.7000010
CrossRef - BeMiller J.N. An introduction to pectins: structure and properties. ACS Symp Ser. 1986;310:37–41. doi: 10.1021/bk-1986-0310.ch001
CrossRef - Caffall K.H, Mohnen D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res. 2009;344:1879–1900. doi: 10.1016/j.carres.2009.05.021
CrossRef - Cantarel B.I, Coutinho P.M, Rancurel C, et al. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2009. doi: 10.1093/nar/gkn663
CrossRef - Canteri-Schemin M.H, Fertonani H.C.R, Waszczynskyj N, Wosiacki G. Extraction of pectin from apple pomace. Brazilian Arch Biol Technol. 2005;48:259–266. doi: 10.1590/S1516-89132005000200013
CrossRef - Collmer A, Bateman D.F. Regulation of extracellular pectate lyase in Erwinia chrysanthemi: evidence that reaction products of pectate lyase and exo-poly-??-d-galacturonosidase mediate induction on d-galacturonan. Physiol Plant Pathol. 1982;21:127–139. doi: 10.1016/0048-4059(82)90032-7
CrossRef - Cosgrove D.J. Growth of the plant cell wall. Nat Rev Mol Cell Biol. 2005;6:850–861. doi: 10.1038/nrm1746
CrossRef - Daher F.B, Braybrook S.A. How to let go: pectin and plant cell adhesion. Front Plant Sci. 2015. doi:10.3389/fpls.2015.00523
CrossRef - Dinu D, Nechifor M.T, Stoian G, et al. Enzymes with new biochemical properties in the pectinolytic complex produced by Aspergillus niger MIUG 16. J Biotechnol. 2007;131:128–137. doi: 10.1016/j.jbiotec.2007.06.005
CrossRef - Dominiak M, Søndergaard K.M, Wichmann J, et al. Application of enzymes for efficient extraction, modification, and development of functional properties of lime pectin. Food Hydrocoll. 2014;40:273–282. doi: 10.1016/j.foodhyd.2014.03.009
CrossRef - Dominiak M.M, Mikkelsen J.D, Marie Søndergaard K. A novel perspective on pectin extraction. 2014.
- Dubey A.K, Yadav S, Kumar M, et al. (2010) In silico characterization of pectate lyase protein sequences from different source organisms. Enzyme Res. 2010:950230. doi: 10.4061/2010/950230
CrossRef - Garg G, Singh A, Kaur A, et al Microbial pectinases: an ecofriendly tool of nature for industries. 3 Biotech. 2016;6:1–13. doi: 10.1007/s13205-016-0371-4
CrossRef - Gonzalez-Candelas L, Kolattukudy P.E. Isolation and analysis of a novel inducible pectate lyase gene from the phytopathogenic fungus Fusarium solani f. sp. pisi (Nectria haematococca, mating population VI). J Bacteriol. 1992;174:6343–6349.
CrossRef - Grenier A.M, Duport G, Pagès S, et al. The phytopathogen Dickeya dadantii (Erwinia chrysanthemi 3937) is a pathogen of the pea aphid. Appl Environ Microbiol. 2006;72:1956–1965. doi: 10.1128/AEM.72.3.1956-1965.2006
CrossRef - Harholt J, Suttangkakul A, Vibe Scheller H. Biosynthesis of Pectin1. Plant Physiol. 2010;153:384–395. doi: 10.1104/pp.110.156588
CrossRef - Hoondal G, Tiwari R, Tewari R, et al. Microbial alkaline pectinases and their industrial applications: A review. Appl Microbiol Biotechnol. 2002;59:409–418. doi: 10.1007/s00253-002-1061-1
CrossRef - Hugouvieux-Cotte-Pattat N, Condemine G, Shevchik V.E. Bacterial pectate lyases, structural and functional diversity. Environ. Microbiol. Rep. 2014;6:427–440.
CrossRef - Hurlbert J.C, Preston J.F. Functional implications of the beta-helical protein fold: differences in chemical and thermal stabilities of Erwinia chrysanthemi EC16 pectate lyases B, C, and E. Arch Biochem Biophys. 2000;381:264–72. doi: 10.1006/abbi.2000.1982
CrossRef - Iglesias M.T, Lozano J.E. Extraction and characterization of sunflower pectin. J Food Eng. 2004;62:215–223. doi: 10.1016/S0260-8774(03)00234-6
CrossRef - Ismail N.S.M, Ramli N, Hani N.M, Meon Z. Extraction and characterization of pectin from dragon fruit (Hylocereus polyrhizus) using various extraction conditions. Sains Malaysiana. 2012;41:41–45. doi: 10.3303/CET1756135
- Kauppinen S, Christgau S, Kofod L V., et al. Molecular cloning and characterization of a rhamnogalacturonan acetylesterase from Aspergillus aculeatus. Synergism between rhamnogalacturonan degrading enzymes. J Biol Chem. 1995;270:27172–27178. doi: 10.1074/jbc.270.45.27172
CrossRef - Kirk O, Borchert T.V, Fuglsang C.C. Industrial enzyme applications. Curr Opin Biotechnol. 2002;13:345–351. doi: 10.1016/S0958-1669(02)00328-2
CrossRef - Kluskens L.D, van Alebeek G-J.W.M, Voragen A.G.J, et al. Molecular and biochemical characterization of the thermoactive family 1 pectate lyase from the hyperthermophilic bacterium Thermotoga maritima. Biochem .J. 2003;370:651–9. doi: 10.1042/BJ20021595
CrossRef - Kumar V, Sangwan P, Singh D, Gill P.K. Global scenario of industrial enzyme market. Nov Publ New York. 2014;91–11. doi: 10.13140/2.1.3599.0083
- Kusuma M.P, Sri Reddy R.D. Thermoalkaline polygalacturonases – a review. Int J Pharm Sci Rev Res. 2014;28:162–165.
- Lara-Márquez A, Zavala-Páramo M.G, López-Romero E, et al. Cloning and characterization of a pectin lyase gene from Colletotrichum lindemuthianum and comparative phylogenetic/structural analyses with genes from phytopathogenic and saprophytic/opportunistic microorganisms. BMC Microbiol. 2011;11:260. doi: 10.1186/1471-2180-11-260
CrossRef - Laskowski R.A. PDBsum: summaries and analyses of PDB structures. Nucleic Acids Res. 2001;29:221–2. doi: 10.1093/nar/29.1.221
CrossRef - Latarullo M.B.G, Tavares E.Q.P, Maldonado G.P, et al. Pectins, Endopolygalacturonases, and Bioenergy. Front Plant Sci.
- 2016;7:1–7. doi: 10.3389/fpls.2016.01401
CrossRef - Lefsih K, Giacomazza D, Dahmoune F, et al. Pectin from Opuntia ficus indica: Optimization of microwave-assisted extraction and preliminary characterization. Food Chem. 2017;221:91–99. doi: 10.1016/j.foodchem.2016.10.073
CrossRef - Leroux J, Langendorff V, Schick G, et al. Emulsion stabilizing properties of pectin. Food Hydrocolloids. 2003; 455–462
CrossRef - Lerouxel O, Cavalier D.M, Liepman A.H, Keegstra K. Biosynthesis of plant cell wall polysaccharides – a complex process. Curr. Opin. Plant Biol. 2006;9:621–630.
CrossRef - Li X, Wang H, Zhou C, et al. Cloning, expression and characterization of a pectate lyase from Paenibacillus sp. 0602 in recombinant Escherichia coli. BMC Biotechnol. 2014;14:18. doi: 10.1186/1472-6750-14-18
CrossRef - Liu Y, Shi J, Langrish T.A.G. Water-based extraction of pectin from flavedo and albedo of orange peels. Chem Eng J. 2006;120:203–209. doi: 10.1016/j.cej.2006.02.015
CrossRef - Liu Y, Shi L, Cheng D, He Z. Dissolving pulp market and technologies: Chinese prospective – a mini-review. BioResources. 2016;11:7902–7916. doi: 10.15376/biores.11.3.Liu
- Malinovsky F.G, Fangel J.U, Willats W.G.T.The role of the cell wall in plant immunity. Front Plant Sci. 2014. doi: 10.3389/fpls.2014.00178
CrossRef - Marín-Rodríguez M.C, Orchard J, Seymour G.B. Pectate lyases, cell wall degradation and fruit softening. J Exp Bot. 2002;53:2115–2119. doi: 10.1093/jxb/erf089
CrossRef - Martens-Uzunova E.S, Schaap P.J. Assessment of the pectin degrading enzyme network of Aspergillus niger by functional genomics. Fungal Genet Biol. 2009.doi: 10.1016/j.fgb.2008.07.021
CrossRef - May C.D. Industrial pectins: Sources, production and applications. Carbohydr Polym. 1990;12:79–99. doi: 10.1016/0144-8617(90)90105-2
CrossRef - Methacanon P, Krongsin J, Gamonpilas C. Pomelo (Citrus maxima) pectin: Effects of extraction parameters andits properties. Food Hydrocoll. 2014;35:383–391. doi: 10.1016/j.foodhyd.2013.06.018
CrossRef - Miller G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030
CrossRef - Mohnen D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008;11:266–277.
CrossRef - Munarin F, Tanzi M.C, Petrini P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012;51:681–689.
CrossRef - Ndeh D, Rogowski A, Cartmell A, et al. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature. 2017;544:65–70. doi: 10.1038/nature21725
CrossRef - Palin R, Geitmann A. The role of pectin in plant morphogenesis. BioSystems. 2012;109:397–402. doi: 10.1016/j.biosystems.2012.04.006
CrossRef - Pedrolli D.B, Monteiro A.C, Gomes E, Carmona E.C. Pectin and Pectinases: Production, Characterization and Industrial Application of Microbial Pectinolytic Enzymes. Open Biotechnol J. 2009;3:9–18. doi: 10.2174/1874070700903010009
CrossRef - Pijning T, van Pouderoyen G, Kluskens L, et al. The crystal structure of a hyperthermoactive exopolygalacturonase from Thermotoga maritima reveals a unique tetramer. FEBS Lett. 2009;583:3665–3670. doi: 10.1016/j.febslet.2009.10.047
CrossRef - Plastow G.S. Molecular cloning and nucleotide sequence of the pectin methyl esterase gene of Erwinia chrysanthemi B374. Mol Microbiol. 1988;2:247–254. doi: 10.1111/j.1365-2958.1988.tb00026.x
CrossRef - Praveen K.G&, Suneetha V. Microbial pectinases : Wonderful enzymes in fruit juice clarification. Int J Medipharm Res. 2016;2:119–127.
- Priya V, Sashi V. Pectinase enzyme producing Microorganisms. Int J Sci Res Publ. 2014;4:2250–3153.
- Remoroza C, Wagenknecht M, Gu F, et al. A Bacillus licheniformis pectin acetylesterase is specific for homogalacturonans acetylated at O-3. Carbohydr Polym. 2014;107:85–93. doi: 10.1016/j.carbpol.2014.02.006
CrossRef - Rose J.K.C. The Plant Cell Wall. 2003.
- Scheller HV, Jensen JK, Sørensen SO, et al (2007) Biosynthesis of pectin. Physiol. Plant. 129:283–295.
CrossRef - Sciences B, Hassan B, Ali S (2016) a Review on Biotechnological Impact of Pectinases in Industries. 1–16.
- Searle-van Leeuwen MJF, van den Broek LAM, Schols HA, et al (1992) Rhamnogalacturonan acetylesterase: a novel enzyme from Aspergillus aculeatus, specific for the deacetylation of hairy (ramified) regions of pectins. Appl Microbiol Biotechnol 38:347–349. doi: 10.1007/BF00170084
CrossRef - Sharma BR, Naresh L, Dhuldhoya NC, et al (2006) An Overview on Pectins. Times food Process J 51:44–51.
- Sharma N, Rathore M, Sharma M (2013) Microbial pectinase: Sources, characterization and applications. Rev. Environ. Sci. Biotechnol. 12:45–60.
CrossRef - Shevchik VE, Hugouvieux-Cotte-Pattat N (1997) Identification of a bacterial pectin acetyl esterase in Erwinia chrysanthemi 3937. Mol Microbiol 24:1285–1301. doi: 10.1046/j.1365-2958.1997.4331800.x
CrossRef - Shevchik VE, Hugouvieux-Cotte-Pattat N (2003) PaeX, a second pectin acetylesterase of Erwinia chrysanthemi 3937. J Bacteriol 185:3091–3100. doi: 10.1128/JB.185.10.3091-3100.2003
CrossRef - Sriamornsak P (2003) Chemistry of pectin and its pharmaceutical uses: a review. Silpakorn Univ Int J 3:207–228. doi: 10.5458/jag.54.211
CrossRef - Srivastava P, Malviya R (2011) Sources of pectin, extraction and its applications in pharmaceutical industry – an overview. Indian J Nat Prod Resour 2:10–18.
- Su H, Qiu W, Kong Q, et al (2015) Thermostable pectate lyase from Caldicellulosiruptor kronotskyensis provides an efficient addition for plant biomass deconstruction. J Mol Catal B Enzym 121:104–112. doi: 10.1016/j.molcatb.2015.08.013
CrossRef - Tapre AR, Jain RK (2014) Pectinases: Enzymes for fruit processing industry. Int Food Res J 21:447–453.
- Thakur BR, Singh RK, Handa AK, Rao MA (1997) Chemistry and uses of pectin — A review. Crit Rev Food Sci Nutr 37:47–73. doi: 10.1080/10408399709527767
CrossRef - Tsuyumu S, Kimura S, Hirata H (2014) Regulation of pathogenicity-related genes in phytopathogenic bacteria and plant. Japan Agric. Res. Q. 48:105–109.
CrossRef - Turner P, Mamo G, Karlsson EN (2007) Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb Cell Fact 6:9. doi: 10.1186/1475-2859-6-9
CrossRef - Wikiera A, Mika M, Starzyńska-Janiszewska A, Stodolak B (2015) Application of Celluclast 1.5L in apple pectin extraction. Carbohydr Polym 134:251–257. doi: 10.1016/j.carbpol.2015.07.051
CrossRef - Willats WGT, Mccartney L, Mackie W, Knox JP (2001) Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 47:9–27.
CrossRef - Wolf S, Greiner S (2012) Growth control by cell wall pectins. Protoplasma 249:169–175.
CrossRef - Xiao C, Anderson CT (2013) Roles of pectin in biomass yield and processing for biofuels. Front Plant Sci. doi: 10.3389/fpls.2013.00067
CrossRef - Xie F, Murray JD, Kim J, et al (2012) Legume pectate lyase required for root infection by rhizobia. Proc Natl Acad Sci 109:633–638. doi: 10.1073/pnas.1113992109
CrossRef - Xu Y, Zhang L, Bailina Y, et al (2014) Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. J Food Eng 126:72–81. doi: 10.1016/j.jfoodeng.2013.11.004
CrossRef - Yadav D (2016) Molecular Biology of Microbial Pectate Lyase : A Review. Br Biotechnol J 13:1–26. doi: 10.9734/BBJ/2016/24893.
CrossRef - Yoder MD, Lietzke SE, Jurnak F (1993) Unusual structural features in the parallel β-helix in pectate lyases. Structure 1:241–251. doi: 10.1016/0969-2126(93)90013-7
CrossRef
(Visited 2,547 times, 1 visits today)

This work is licensed under a Creative Commons Attribution 4.0 International License.






