Manuscript accepted on : 16 November 2017
Published online on: --
Plagiarism Check: Yes
Isolation, Purification and Application of Secondary Metabolites from Lichen Parmelia Perlata
PG and Research Department of Biotechnology, Women’s Christian college, College road, Chennai - 600006, Tamilnadu, India.
Corresponding Author E-mail: dr.anchanababu@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2587
ABSTRACT: Lichens are composite algae having a symbiotic association with a fungal partner. They produce numerous secondary metabolites, which play an important role in pharmaceutical and in other industrial applications. The Secondary metabolites produced by lichens are found to be 80% more when compared to that produced by other organisms. Not much work has been carried out on lichens due to the difficulty in their cultivation but still it emerges as a potential source in developing therapeutically important drugs which are widely beneficial in all fields of application. The Present study was aimed to isolate, purify and determine the applications of secondary metabolites from Lichen Parmelia perlata. The presence of these compounds were detected and purified by thin layer chromatography and column chromatography using specific solvent systems. The purified fractions were then identified by Gas chromatography-Mass spectrometry (GC-MS). The compounds were then subjected to application oriented studies such as antimicrobial activity, antioxidant activity and antidiabetic activity. Not much work have been carried out on the isolation of a specific glycoside and alkaloid compound from Lichen Parmelia perlata, so this study was an attempt to explore the applications of these individual compounds which could prove beneficial to the mankind for different purposes.
KEYWORDS: Column Chromatography; Gas Chromatography-Mass Spectrometry Parmelia Perlata; Secondary Metabolites; Thin Layer Chromatography;
Download this article as:| Copy the following to cite this article: Leela K, Devi A. C. Isolation, Purification and Application of Secondary Metabolites from Lichen Parmelia Perlata. Biosci Biotech Res Asia 2017;14(4). |
| Copy the following to cite this URL: Leela K, Devi A. C. Isolation, Purification and Application of Secondary Metabolites from Lichen Parmelia Perlata. Biosci Biotech Res Asia 2017;14(4). Available from: https://www.biotech-asia.org/?p=28294 |
Introduction
Lichens are considered to be a symbiotic association between a fungal and a photosynthetic partner which is usually an algae or Cyanobacterium. They are widely distributed in all terrestrial habitats and are found to grow inside rocks, on wood, soil, on other lichens, on glass, metals and plastics (Joneson and Lutzoni, 2009). Lichens produce numerous secondary metabolites which are deposited on the surface of fungal hyphae in the form of tiny crystals and these metabolites have enormous applications and are widely utilized for different purposes. Usnic acid is an active metabolite found in the genus of Usnea with applications in pharmaceutical preparation, antiviral, antimicrobial and analgesic activity (Proksa and Proksova, 1999). Pulvinic acid is another metabolite isolated from various lichens and fungi which are found to possess antioxidant properties (Fournet et al., 1997). Lichens thus play an important role and hence are widely utilized for different applications such as in cosmetics, food, in diagnosis of diseases such as eczema, arthritis and respiratory disorders etc.
Parmelia perlata (Huds) Ach (family: Parmeliaceae) is a species of lichen that is generally referred to as “Stone flower or Charila” (Kritikar KR and Basu BD, 1987). It is generally distributed on the surface of walls, old trees and also found extensively in hilly areas of Indian subcontinent. They are also used as spice to improve the flavour of food (Bhattarai T et al., 1999). Parmelia perlata was found to contain numerous secondary metabolites such as atranorin, salazinic acid, Protolichesterinic acid, lecanoric acid and are used as bioindicators of heavy metal pollution (Momoh MA and Adikwu MU, 2008). Parmelia perlata was found to possess innumerable applications in medical sciences such as antiemetic, analgesic and antipyretic activities. Due to its important medicinal properties it is widely utilized in treating boils, inflammations, sores, seminal weakness and amenorrhoea. A compound gyrophoric acid isolated from Parmelia species was found to be an inhibitor of growth of human keratinocytes and was also reported to exhibit antimitotic, antitumor activities (K. Muller., 2001).
Secondary metabolites are organic molecules that do not play a role in growth and development of an organism. They are widely synthesized by various natural sources such as bacteria, fungi, algae, plants and animals. The secondary metabolites are mainly classified on the basis of their biosynthetic origin. They play an important role in medicinal, colorant, aromatic and functional foods. They are classified into three main groups: Terpenes, Phenolic compounds and Nitrogen containing compounds. In the current study two different secondary metabolites have been targeted and isolated they are Glycosides and Alkaloids.
Glycosides are organic compounds which on hydrolysis yield one or more sugar moieties along with the non sugar moiety. Sugar moiety is referred to as glycon and non sugar moiety is called as aglycone. Glycoside is classified into different groups based on chemical nature of Non-sugar moiety, based on the nature of sugar moiety, based on linkage between glycon and aglycone portion and based on their therapeutic nature. (Fig 1)
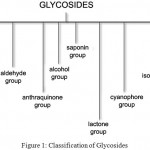 |
Figure 1: Classification of Glycosides
|
Alkaloids are naturally occurring compounds containing nitrogen atoms. The name was derived from word alkaline which denotes a nitrogen containing base. These compounds are produced by a number of organisms such as bacteria, fungi, plants as well as animals. The alkaloids are also widely utilized as medications, recreational drugs etc. Alkaloids are generally classified into the following groups such as pyridine, pyrrolidine, Tropane, Indolizidine, Quinoline, Isoquinoline, Phenanthrene, Phenethylamine, Purine and Terpenoid group. (Fig 2)
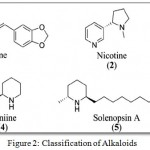 |
Figure 2: Classification of Alkaloids
|
Thus in the present study the antimicrobial, antioxidant and antidiabetic applications of Glycoside and Alkaloid compounds isolated and purified from the Lichen Parmelia perlata were determined.
Materials and Methods
Sample Preparation
Dried samples of Lichen Parmelia perlata were obtained from Chennai, India and were authenticated based on the morphological and relevant keys given in the literature (Aswathi DD, 1988). The sample was cleaned, washed under tap water and distilled water to remove the dirt, it was dried and powdered using a mixer. The powdered lichen sample was stored in clean bottle for further analysis.
Extraction of Samples: (Rashmi S. and Rajkumar H.G, 2014)
The crude extract from the Lichen samples were obtained by means of cold extraction method using methanol as solvent. About 50g of the powdered lichen sample was added to 500ml of methanol in a conical flask, covered with aluminum foil and kept on a rotary shaker for 3 days at room temperature. The solution was filtered with the help of Whatman No.1 filter paper and the filtrate obtained was evaporated. The dried extracts were then stored for further experiments. The yield of respective crude extract was calculated as:
Percentage yield (%) = (dry weight of extract/dry weight of samples) × 100.
Identification, Isolation and Purification of Secondary Metabolites
Thin Layer Chromatography
Thin-layer chromatography (TLC) is a chromatographic technique used for separating different components from a mixture. It is generally carried out on a thin sheet of plastic, glass or an aluminium foil which is coated with an absorbent material. The adsorbent could be cellulose, silica or aluminium oxide (alumina). The layer of adsorbent material is called as stationary phase. The sample is spotted on the plate and the solvent or mixture of solvents acts as a mobile phase via capillary action. TLC plate is cut and using a pencil line is drawn about 1cm from one end of the edge. Markings are made and the samples were spotted on to the plate using a capillary tube for each spot. The spots should be air dried. The TLC chamber consists of a glass jar with a lid and the solvent mixture is added to chamber. The Mobile phase used for isolation of glycosides are Toluene: methanol: glacial acetic acid: water = 7:4:3:1 and for alkaloids are Butanol: acetic acid: water = 4:1:3 (Viseshni R et al., 2017). The TLC plates were placed carefully in the TLC chamber. The solvent mixture should be below the spot on the plate and the chamber is kept closed. As the solvent moves up due to capillary movement, the compounds present in the extract are separated. The TLC plates are then removed from the chamber once the solvent reaches an approximate level in the plate and is kept for drying. The dried plates are observed for spots by using a suitable spraying reagent. For glycosides Iodine chamber is used while for alkaloids Dragendroff’s reagent is used for identification (Kumar et al., 2007) (Gurpreet kaur et al., 2014). The formation of brown band indicates the presence of glycosides and the formation of reddish orange band indicates the presence of alkaloids. The Rf value is calculated using the formula:
Rf = Distance travelled by the solute / Distance travelled by the solvent
Column Chromatography: (G.K.Jayaprakasha et al., 1998)
Silica gel (100 – 200 mesh) was chosen as the stationary phase. The column was packed with silica gel using methanol once packed the crude residue from methanol extract was transferred onto the bed of silica gel. The column was run by using mobile phase (Toluene: methanol: glacial acetic acid: water in the ratio of 35:20:15:10) for glycosides and (Butanol: acetic acid: water in the ratio of 40:10:30) for alkaloids. The fractions were collected at an interval of 5ml each and are monitored by means of thin layer chromatography. The fractions obtained were stored and utilized for the identification of individual compounds present in the sample by means of Gas chromatography – Mass spectrometry (GC-MS).
Confirmatory Test: (Sibi G et al., 2013)
Glycosides: Keller –Killani Test: To 1ml of sample add 1ml of glacial acetic acid and 1ml of concentrated Sulphuric acid. Appearance of reddish brown colour at the junction of 2 layers indicates the presence of glycosides.
Alkaloids: Dragendroff’s test: To 2ml of sample 2-3 drops of Dragendroff’s reagent is added. Appearance of orange red coloured complex indicates the presence of alkaloids
Identification of Secondary Metabolites
Gas Chromatography – Mass Spectrometry (GC-MS): (Ruthiran papitha et al., 2017)
It is an analytical method used for identifying different substances within a test sample. It also helps in identifying trace elements in a sample. The Clarus 680 GC was used for the analysis and a fused silica column was employed and packed with Elite-5MS (5% biphenyl 95% dimethylpolysiloxane, 30 m × 0.25 mm ID × 250μm df) and the components were separated using Helium as carrier gas at a constant flow of 1 ml/min. The injector temperature was set at 260°C during the chromatographic run. The 1μL of extract sample was injected into the instrument and the oven temperature was as maintained as follows: 60°C (2 min); followed by 300°C at the rate of 10°C min−1; and 300°C, where it was held for 6 min. The mass detector conditions were: transfer line temperature 240°C; ion source temperature 240°C; and ionization mode electron impact at 70 eV, a scan time 0.2 sec and scan interval of 0.1 sec. The fragments were obtained from 40 to 600 Da. The spectrums of the components were compared with the database of spectrum of known components stored in the GC-MS NIST (2008) library.
Applications
Antimicrobial Activity
The antibacterial activity of Purified glycoside and Purified alkaloid fractions of Lichen (Parmelia perlata) were screened against both Gram positive and Gram negative bacteria such as Staphylococcus aureus, Streptococcus spp, Escherichia coli, Klebsiella pneumoniae, Salmonella spp and Pseudomonas aeruginosa while the antifungal activity was carried out against Aspergillus spp and Candida albicans.
Agar Well Diffusion Method: (K.Nanthini Devi et al., 2012)
The stock cultures were maintained at 4°C on the nutrient agar slant slopes. Nutrient broth was prepared for about 50ml and a loop-full of stock cultures were transferred to 50ml of nutrient broth and were incubated at 37°C for 24 hours. The strains were inoculated in nutrient broth for 24 hours. Potato dextrose broth was prepared for the growth of fungal strains and incubated for 48 hours. About 250ml of Muller Hinton agar medium (MHA) was prepared for antibacterial activity while Potato Dextrose agar medium (PDA) was prepared for antifungal activity and poured into the petriplates and was allowed to solidify. Once solidified the bacterial cultures and the fungal cultures were swabbed onto the agar medium using a sterile cotton swab .The wells were punctured using a sterile cork borer. Different concentrations of samples (20, 40, 60, 80mg/ml) were dispensed into each well using a micropipette. The petriplates were then incubated at 37°C for 24 hours for bacteria and 37°C for 3-4 days for fungi and observed for the zone of inhibition. The diameter of the zone of inhibition was measured in mm.
Antioxidant Activity
The antioxidant potential of Purified glycoside and Purified alkaloid fractions of lichen (Parmelia perlata) were determined by 3 methods: Total antioxidant capacity (TAC) assay, Hydrogen Peroxide (H2O2) scavenging assay and reducing power assay
Total Antioxidant Capacity Assay
About 1ml of the test sample is added to 3ml of freshly prepared phosphomolybdenum reagent and was incubated for about 90 minutes in water bath at 95ºC .It was then cooled to room temperature and absorbance was measured at 695nm in a spectrophotometer.1ml methanol without extract was used as blank. Ascorbic acid was used as the standard (Prieto et al., 1999).The Total antioxidant capacity (TAC) can be calculated using the formula:
Total antioxidant capacity (TAC) = (A of Control – A of test) / A of Control × 100
Hydrogen Peroxide Scavenging Assay
Solution of Hydrogen peroxide (40mM) was prepared in phosphate buffer (pH 7.4).1ml of the test sample was added to 3ml of Hydrogen peroxide solution and was incubated at room temperature for 10 minutes and the absorbance was determined at 230nm in a spectrophotometer. Ascorbic acid was used as the standard. Phosphate buffer without hydrogen peroxide served as blank (Ruch et al., 1989).It can be calculated using the formula:
% scavenged (H2O2) = (A of Control – A of test / A of Control) × 100
Reducing Power Assay
In this method 2.5ml of sample was mixed with 2.5ml of phosphate buffer (0.2M, pH 6.6) and 2.5ml of 1% Potassium ferricyanide (10mg/ml).The mixture was incubated at 50ºc for 20 minutes then rapidly cooled and mixed with 2.5ml of 10% trichloroacetic acid and centrifuged at 6500 rpm for 10 minutes. About 2.5ml of supernatant was diluted with 2.5ml of distilled water and then 0.5ml of 0.1% ferric chloride was added and allowed to stand for 10 minutes. The absorbance was read spectrophotometrically at 700nm (Ferreira et al., 2007).
Antidiabetic Activity: (Murugesan S et al., 2016)
The antidiabetic activity of Purified glycoside and Purified alkaloid fractions of lichen (Parmelia perlata) were determined by means of alpha amylase inhibition assay.
Alpha Amylase Inhibition Assay
About 1ml of the sample was added to 1ml starch solution and was incubated for 10 minutes at room temperature.0.5ml of the prepared enzyme solution was added to the mixture and was incubated at 25ºc for about 10 minutes. The reaction was then terminated by the addition of 1ml of colorimetric reagent and was kept in water bath for 5 minutes and cooled to room temperature. This was further diluted by adding 10ml of distilled water and the absorbance of the mixture was measured at 540nm in colorimeter. Sample without extract served as blank. The % inhibition was calculated using the formula:
% inhibition = (A of Control – A of test / A of Control) × 100
Results and Discussion
Extraction
The Lichen (Parmelia perlata) sample was extracted by means of cold extraction method using methanol. Methanol has a polarity index of 5.1 and was found to be capable of dissolving polar compounds. Therefore methanol was highly preferred and the solvent was also reported to have been used by other authors for their extraction purposes (Hafizur Rahman et al., 2014). The Percentage yield of Lichen crude extract was found to be 2.56 % and it has been reported to be the total yield for about 50 grams of the dry weight of the sample (A.Mastan et al., 2014). The work by Fadwa Ali Mohamed Abdullah and Mona M.A. Abdalmageed, 2016 have reported the extraction of Lichen Parmelia perlata using petroleum ether solvent and the total yield was found out to be 0.27 %
Identification of Secondary Metabolites – Thin Layer Chromatography
Thin layer chromatography is a technique used for the identification of secondary metabolites in a sample by using the solvents in different ratios. In this study thin layer chromatography was employed to identify the presence of Glycosides and Alkaloid compounds in the Lichen sample. For Glycosides the mobile phase used is Toluene: methanol: glacial acetic acid: water = 7:4:3:1 and the bands were visualized by placing it in the iodine chamber while for Alkaloids the mobile phase used is Butanol: acetic acid: water = 4:1:3 and the bands were visualized by spraying it with Dragendroff’s reagent (Fig 3). The solvent systems were used based on the paper Viseshni R et al., 2017
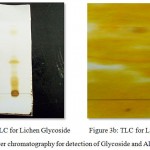 |
Figure 3a,b: Thin Layer chromatography for detection of Glycoside and Alkaloid compounds
|
Table 1: Rf Values of the Glycoside and Alkaloid bands of Lichen
<
| S.NO | COMPOUNDS | Rf VALUES |
| 1 | Glycoside | 0.325, 0.20, 0.15 |
| 2 | Alkaloid | 0.60, 0.526 |
The Rf values obtained for the TLC detection of glycoside compound was found to be 0.325, 0.20, 0.15 (Ravindra C. Sutar et al., 2014) Similarly the Rf values obtained for the TLC detection of alkaloid compound was found to be 0.60, 0.526 (Karthikeyan S et al., 2013) (Table 1).
Isolation of Secondary Metabolite Fractions using Column Chromatography
Column chromatography is performed in order to purify the individual compounds present in the sample from a mixture of compounds. The purified fractions collected by means of Column chromatography were sent for GC-MS (Gas chromatography – Mass spectrometry) analysis in order to identify the major secondary metabolites or the compounds present in the purified sample. The Stationary phase is Silica gel (100 – 200 mesh) while Mobile phase used for Glycosides – Toluene: methanol: glacial acetic acid: water = 35:20:15:10 and for Alkaloids – Butanol: acetic acid: water = 40:10:30.
 |
Figure 4a,b,c,d: Purified Glycoside and Alkaloid fractions obtained through column chromatography
|
Totally two glycoside and two alkaloid fractions were obtained through column chromatography. Among both the fractions the Glycoside fraction I and Alkaloid fraction I (Fig 4a and 4c) was subjected to confirmation by performing Keller-Killani test and Dragendroff’s test to detect the presence of glycoside and alkaloid compounds in that particular fraction and TLC was also carried out and the corresponding Rf Values were recorded.
Table 2: Rf value of the Purified Glycoside and Alkaloid fractions of Lichen Parmelia perlata
| S.No | Compounds | Rf Value |
| 1 | Glycoside | 0.125 |
| 2 | Alkaloid | 0.526 |
The Rf values obtained for the TLC of individual purified fractions were found to be 0.125 and 0.526 (Table 2). The values were compared with reference using various literatures and are considered to be glycoside and alkaloid compounds respectively (Ravindra C. Sutar et al., 2014) (S.K. Reshmi et al., 2010)
 |
Figure 5a,b: Confirmatory test performed for the purified Glycoside and Alkaloid fractions I
|
The appearance of Reddish brown ring at the junction of two layers indicates the presence of Glycoside and the formation of orange red coloured complex indicates the presence of Alkaloid in the Lichen sample (Fig 5).
Gas Chromatography – Mass Spectroscopy (GC-MS)
The Purified fractions (Glycoside fraction I and Alkaloid fraction I) were then subjected to identification by Gas chromatography-Mass spectrometry. It is an analytical method used for identifying different compounds within a test sample. In this study Glycoside and alkaloid compounds isolated through column chromatography were identified by means of GC-MS technique. The acquisition parameters followed for GC-MS analysis: Oven: Initial temp 60°C for 2 min, ramp 10°C/min to 300°C, hold 6 min, Total Run Time: 32.00 mint InjA auto=260°C, Volume=1 μL , Split=10:1,Flow Rate: 1 mL/mint, Carrier Gas=He, Column=Elite-5MS (30.0m, 0.25mmID, 250μm df).
Lichen Glycoside
 |
Figure 6a: GC-MS Chromatogram of Lichen Glycoside fraction showing various peaks at different retention time
|
GC-MS analysis revealed the presence of 7 different compounds in the fraction at different retention times such as 3,5-dihydroxytoluene (14.933), Benzoic acid,2,4-dihydroxy-6-methyl-, methyl ester (16.754), Methyl 4-Methoxy Salicylate (16.924), Acetic acid,(3,4-Dimethyl-5-oxo-2(5H)-Furanylidene)-, Methyl ester (18.264), 1-Methyl Heptyl Trans-2,2-Dimethyl-3-(2-methyl-1-propenyl) cycloprop (19.585), 1-Hexyl-2-Nitrocyclohexane (21.171), 6-Nitroundec-5-ene (21.366) respectively (Fig 6a).
 |
Figure 6b: GC-MS spectrum for Lichen Glycoside (Methyl 4-Methoxy Salicylate)
|
Among the obtained compounds Methyl 4-Methoxy Salicylate with retention time 16.924 was identified to be a glycoside and the GC-MS spectrum for the corresponding glycoside is also given in the Fig 6b. This compound is found to play an important role in pharmaceutical industry as an analgesic and as an antiseptic agent. It is used as a flavouring agent and also in anti herbivore defense. It is also widely employed in immunohistochemistry (Hoang Le Tuan Anh et al., 2017).
Lichen Alkaloid
 |
Figure 6c: GC-MS Chromatogram of Lichen Alkaloid fraction showing various peaks at different retention time
|
GC-MS analysis revealed the presence of 9 different compounds in the fraction at different retention times such as 1,4-Benzenediol,2-Methyl- (17.544), 2,5,9-Tetradecatriene,3,12-Diethyl (18.089), N-(1-Cyclohexen-1-yl) Piperidine (18.325), Pyrrolidine, 1-(1- Cyclohexen-1-yl)- (18.710), P- Dodecyloxybenzaldehyde (19.935), N- Noneyl Succinic anhydride (19.970), 7-Antiacetyl – 3,3- dimethylbicyclo (2.2.2) octan-2-one (20.250), 1-naphthalenepropanol, alpha-ethyldecahydro- 2,4- dihydroxy-AL (20.435), 2-oxabicyclo (4.4.0) Decan – 10 – one, 1,3,7,7-Tetramethyl- (1R,3R,6R) (30.149) (Fig 6c).
 |
Figure 6a,b,c,d: GC-MS analysis of Glycoside and Alkaloid compounds from Lichen Parmelia perlata
|
Among the obtained compounds N-(1-Cyclohexen-1-yl) Piperidine with retention time 18.325 was identified to be an alkaloid and the GC-MS spectrum for the corresponding alkaloid is also given in the Fig 6d. This compound is known as the best representative structural element in alkaloids. It is widely used in the synthesis of organic compounds and also in chemical degradation reactions. It is also employed as an antidepressant agent in pharmaceuticals (Rui Yan et al., 2014).
The compounds identified through GC-MS analysis were then subjected to application oriented studies wherein the antimicrobial, antioxidant and antidiabetic applications of the purified Glycoside and Alkaloid fractions of Lichen Parmelia perlata were assessed.
Antimicrobial Activity
The antibacterial activity of the Lichen glycoside and alkaloid fractions were determined against both the Gram positive and Gram negative bacteria such as Staphylococcus aureus, Streptococcus spp, Escherichia coli, Klebsiella pneumoniae, Salmonella spp and Pseudomonas aeruginosa were used for the study.
Table 3: Antibacterial activity of Lichen Glycoside and Alkaloid fractions at different concentrations
| S.No | Organism | Zone of Inhibition (in mm) | |||||||||
| Lichen Glycoside
Concentration (mg/ml) |
Lichen Alkaloid
Concentration (mg/ml) |
||||||||||
| 20 | 40 | 60 | 80 | C | 20 | 40 | 60 | 80 | C | ||
| 1 | E.coli | 6 | 8 | 9 | 10 | 0 | 2 | 5 | 8 | 12 | 0 |
| 2 | Streptococcus spp. | 7 | 8 | 10 | 14 | 0 | 3 | 5 | 7 | 10 | 0 |
| 3 | Klebsiella Pneumoniae | 5 | 5 | 10 | 12 | 0 | 2 | 4 | 5 | 5 | 0 |
| 4 | Staphylococcus aureus | 4 | 5 | 7 | 9 | 0 | 2 | 5 | 6 | 8 | 0 |
| 5 | Salmonella typhimurium | 3 | 4 | 6 | 7 | 0 | 1 | 2 | 3 | 5 | 0 |
| 6 | Pseudomonas aeruginosa | 3 | 5 | 5 | 6 | 0 | 1 | 3 | 4 | 5 | 0 |
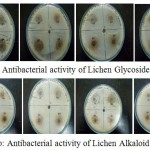 |
Figure 7a,b: Antibacterial activity of Lichen Glycoside and Alkaloid fractions at different concentrations
|
The glycoside and alkaloid fractions were taken at different concentrations (20, 40, 60, 80mg/ml) and were tested for their antibacterial activity against six bacterial organisms. The zone of inhibition was found to increase with increasing concentration of the compounds. Both the glycoside and alkaloid fractions showed good antibacterial activity against all the six bacterial organisms but among the two fractions glycoside fraction was found to be comparatively better showing an increased zone of inhibition than the alkaloid fraction (Table 3). A study by Musaddique Hussain et al., 2014 has reported the antimicrobial potential of lichen Parmelia perlata extracts against different pathogenic microbes wherein the crude extract was found to have shown maximum inhibition against E.coli with zone of inhibition around 21.75mm followed by Pseudomonas aeruginosa (21mm), Staphylococcus aureus (20.25mm), Streptococcus pneumoniae (19.50mm) and Klebsiella pneumoniae (17.90mm) respectively.
The antifungal activity of Lichen glycoside and alkaloid fractions were also determined against 2 fungi Aspergillus spp and Candida albicans.
Table 4: Antifungal activity of Lichen Crude extract, Glycoside and Alkaloid fractions
| S.No | Organism | Zone of Inhibition (in mm) | |||
| Lichen
Crude Extract |
Lichen Glycoside | Lichen Alkaloid | Control | ||
| 1 | Aspergillus niger | 10 | 22 | 15 | 0 |
| 2 | Candida albicans | 11 | 15 | 11 | 0 |
 |
Figure 8: Antifungal activity of Lichen crude extract, Glycoside and Alkaloid fractions
|
The glycoside and alkaloid fractions were tested for their antifungal activity against 2 fungal organisms wherein both the fractions showed a good zone of inhibition against the fungal organisms but comparatively glycoside fraction was found to show much better activity than the alkaloid fraction .The lichen crude extract also exhibited good antifungal activity but was found to be comparatively low than that of the individual purified fractions (Table 4). A study by Musaddique Hussain et al., 2014 has also reported the antifungal potential of lichen Parmelia perlata extracts against 2 pathogenic fungal organisms wherein the crude extract was found to have shown maximum inhibition against Candida albicans with inhibition zone around 14.25mm followed by Aspergillus niger with zone around 13.50mm respectively.
Antioxidant Activity
The antioxidant activity of the Lichen crude extract, Purified glycoside and alkaloid fractions were carried out by means of three assays: Total antioxidant capacity (TAC) assay, Hydrogen peroxide scavenging (H2O2) assay and reducing power assay.
Total Antioxidant Capacity (TAC) Assay
It is a widely employed technique for detecting the antioxidant potential of the biological samples. The amount of free radicals scavenged by the test solution can be measured. This assay is carried out by means of phosphomolybdenum method. This technique is based on the principle of reduction of Phosphate -Mo (VI) to Phosphate – Mo (V) by the test sample and the immediate formation of bluish green coloured Phosphate/Mo complex at an acidic pH. It has several advantages such as low cost, increased rate of reaction and this method can also be performed using automated or manual methods (Rohan Sharadanand Phatak and Anup Subash Hendre, 2014)
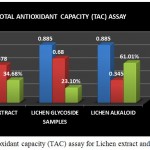 |
Figure 9: Total antioxidant capacity (TAC) assay for Lichen extract and purified fractions
|
The Total antioxidant capacity (TAC) of the lichen crude extract and the individual purified fractions were determined. In case of TAC assay both glycoside and alkaloid compounds showed good antioxidant percentage but on comparison alkaloid compound was found to have shown a good % of activity than the crude extract and the glycoside fraction (Fig 9). A study by A.Mastan et al., 2014 has reported the total antioxidant capacity of methanolic and aqueous extracts of 4 different lichen samples such as Cladonia fimbriata, Permilopsis ambigua, Punctelia subrudecta and Evernia mesomorpha by phosphomolybdenum method wherein the antioxidant percentage was found to be higher in the methanolic extracts of lichen samples compared to that of the aqueous extract.
Hydrogen Peroxide Scavenging Assay
Hydrogen peroxide is considered to be a weak oxidizing agent. Inside the cell hydrogen peroxide reacts with Fe2+ or Cu2+ ion and gets converted into hydroxyl radical which could cause toxic effects and thereby leads to cell damage. So it is essential to prevent the formation of hydroxyl radical by preventing the accumulation of hydrogen peroxide within the cell. This assay is based on the principle of decrease in absorbance of hydrogen peroxide upon oxidation of hydrogen peroxide. The ability of the extracts to scavenge the hydrogen peroxide is determined (Priyanka B et al., 2013).
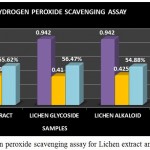 |
Figure 10: Hydrogen peroxide scavenging assay for Lichen extract and purified fractions
|
The Hydrogen peroxide scavenging assay for the lichen crude extract and the individual purified fractions were carried out and determined. In hydrogen peroxide scavenging assay the crude extract as well as the purified fractions showed good scavenging % but on comparing the activity of both glycoside and alkaloid compounds glycosides were found to have shown a better % of scavenging than the alkaloid and crude extract (Fig 10). A study by S.Suganya, 2015 has reported the Hydrogen peroxide scavenging activity of the Petroleum ether, ethyl acetate, ethanol and aqueous extracts of lichen Parmotrema grayanum at different concentrations such as 20, 50, 100, 200µg/ml. Among all the extracts ethanolic extract of the lichen sample was found to have shown increasing scavenging percentage at all concentrations followed by petroleum ether extract respectively.
Reducing Power Assay
It is a widely used technique and is based on the principle that the substances with reduction potential react with Potassium ferricyanide (Fe3+) to form Potassium ferricyanide (Fe2+) which again reacts with ferric chloride to form ferric-ferrous complex that has an absorption at 700nm.The presence of reducing compound in the extract will tend to cause the conversion of ferric form to ferrous form .By measuring the absorbance of blue colour formed at 700nm it is possible to determine the concentration of Fe3+ ion .Thus the reducing ability of the crude extract and individual purified fractions were determined (P. Jayanthi and P.Lalitha.,2011).
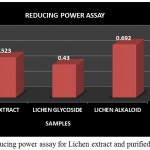 |
Figure 11: Reducing power assay for Lichen extract and purified fractions
|
The Reducing power assay was carried out for the lichen crude extract and the individual purified Glycoside and Alkaloid fractions. In reducing power assay the absorbance of the individual samples were analysed wherein the absorbance of alkaloid fraction was found to be comparatively higher than that of the glycoside fraction and the crude extract but overall the crude extract as well as both the purified fractions showed an increased activity (Fig 11). High absorbance of the extract as well as fractions indicates potent reducing power. A study by A.Mastan et al., 2014 has reported the reducing power assay for the methanolic and aqueous extracts of 4 different lichen samples such as Cladonia fimbriata, Permilopsis ambigua, Punctelia subrudecta and Evernia mesomorpha wherein the value of absorbance was found to vary from 0.12 to 1.98. All the 4 lichen samples were found to have shown increased absorbance but among theses lichen extracts aqueous extract of lichen Evernia mesomorpha was found to have shown better antioxidant activity with an increased absorbance.
Antidiabetic Activity
The antidiabetic activity of the crude extract and purified Glycoside as well as Alkaloid fractions of Lichen Parmelia perlata were determined by means of alpha amylase inhibition assay. It is based on the principle of in vitro hydrolysis of starch in the presence of α-amylase enzyme. This process is quantified using iodine which gives blue colour on reaction with starch. The decrease in the intensity of blue colour indicates the breakdown of starch by the enzyme into monosaccharides. If the extracts possess an increased activity then the intensity of blue colour will be more or higher thus the intensity of blue colour developed is directly proportional to the alpha amylase inhibitory activity (Sheikh et al., 2008).
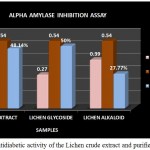 |
Figure 12: Antidiabetic activity of the Lichen crude extract and purified fractions
|
The antidiabetic activity of Lichen Parmelia perlata were determined by means of alpha amylase inhibition assay in which the % inhibition of the crude extracts as well as the purified fractions were analysed. The crude extract as well as the purified compounds showed good inhibition % but among the two fractions glycoside fraction was found to possess higher antidiabetic activity compared to that of the alkaloid fraction and the crude extract (Fig 12). A study by Rashmi shivanna et al., 2015 has reported the antidiabetic activity of methanol and ethyl acetate extracts of 2 lichen samples namely Flavoparmelia caperata and Physcia aipolia at different concentrations such as 5, 10, 15mg/ml using alpha amylase inhibition assay wherein the methanolic extract of Flavoparmelia caperata were found to have shown 49% inhibition followed by Physcia aipolia with 46% inhibition.
Conclusion
The Present study was carried out in order to explore the potential of individual secondary compounds such as glycoside and alkaloid isolated from Lichen Parmelia perlata in different therapeutic applications such as antimicrobial, antioxidant and antidiabetic activity. A number of lichen compounds are being screened and extracted to test their pharmacological potential so that these compounds can also be employed in developing therapeutically important drugs. Since the compounds are derived from natural sources they do not cause any health impacts or don’t pose any threat to the environment. The use of drugs derived from these natural sources could also help in treating a number of diseases due to the accumulation of bioactive compounds. Many of the compounds derived from these natural sources have been found to possess numerous pharmacological properties. Not much work has been reported before on the isolation of glycoside and alkaloid compounds from lichen so this study was an attempt to identify the presence of such beneficial compounds in Lichen that could prove to be useful for different applications.
Acknowledgement
The authors are highly thankful to Dr. Lillian Jasper, Principal, Women’s Christian College and the Department staff members for the needful support.
Conflict of Interest
The authors declared no conflict of interests.
References
- Mastan A, Sreedevi B, Kumari P.J. Evaluation of the in vitro Antioxidant and Antibacterial Activities of Secondary Metabolites produced from Lichens. Asian Journal of Pharmaceutical and Clinical Research. 2014; 7( 1):193-198.
- Aswathi D.D. A key to the macrolichens of India and Nepal, Journal of the Hattori. Botanical Lab. 1988;65:207-302.
- Tribikram B, Dilip S, Rama S. Nutritional value of some edible Lichens of east Nepal. Journal of Applied Botany . 1999;73(1/2):11-14.
- Ali F, Abdullah M, Abdalmageed M.M.A. The phytochemical screening and antimicrobial activity of petroleum ether extract of Parmelia perlata (Huds.) Ach. International Journal of Innovative Pharmaceutical sciences and Research. 2016;4(3):186-195.
- Ferreira I. C, Baptista F.R, Vilas-Boas P. & Barros M, Free-radical L. scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal. Food Chemistry. 2007;100:1511–1516.
CrossRef - Fournet A, Ferreira M.E, deArias R.A, deOrtiz T.S. Inchausti A, Yaluff G, Quilhot W, Fernandez E and Hidalgo M.E. Photoprotector capacity of lichen Comp. Physiol. Pharmacol. Toxicol. Endocrinol. 1997;116:51–54.
- Jayaprakasha G.K, Jaganmohan L.R, Singh.R.P and Sakariah K.K. Improved chromatographic method for the purification of phenolic constituents of Lichen Parmotrema tinctorum (NYl) Hale. Journal of chromatographic science. 1998;36:1018-1022.
CrossRef - kaur G, Pandhair V, Cheema G.S. Extraction and characterization of steviol glycosides from Stevia rebaudiana bertoni leaves. Journal of Medical plant studies. 2014;2(5):41-45.
- Rahman H, Vijaya B,Ghosh S , Pant G, Sibi G . In vitro studies on antioxidant,hypolipidemic and cytotoxic potential of Parmelia perlata, American Journal of Life Sciences. 2014;2(6-1):7-10.
- Le H.T.A,Thi D.D,Thanh D.T, Quy T.H,Thi P.H.Y, Hong T.Q,Xuan N.N ,Van C.M,Thi D.H.Y, Van P.K. Hepatoprotective effects of Phenolic glycosides from methanol extract of Physalis angulate. Journal of Science and Technology. 2017;55(2):161-167.
- Joneson S and Lutzoni F. Revisiting compatibility and thigmotropism in the lichen symbiosis. 2009;47:109–115.
- K . Pharmaceutically relevant metabolites from lichens. Applied Microbiol Biotechnol. 2001;56(1):9-16.
- Devi N.K, kumar A, Marudhupandi T.T K. V and Balasubramanian T.T. Evaluation of antibacterial and antioxidant properties from brown seaweed Sargassum wightii (Greville, 1848) against human bacterial pathogens. International Journal of Pharmacy and Pharmaceutical sciences. 2012;4:143-149.
- Karthikeyan S, Sivakumar A, Anbalagan M, Nalini E and Gothandam K.M. Fingerprinting of Alkaloids, steroids and flavanoids using HPTLC of Leucas aspera L.whole plant methanolic extract. Journal of Pharmaceutical sciences and Research. 2013;5(3):67-71.
- Kiritikar K.R and Basu B.D . Indian Medicinal plants Ed.3. Pub Lalit Basu M.M.B. Allahabad. 1987;1996:2757.
- Kumar S, Jayaveera K.N, Kumar C.K.A, Sanjay U.P, Swamy B.M.V and Kumar D.V.K. Antimicrobial effects of Indian medicinal plants against acne-inducing bacteria. Trop. J. Pharm. Res. 2007;6:717-723.
CrossRef - Momoh M.A, Adikwu M.U. Evaluation of the effect of colloidal silver on the antibacterial activity of ethanolic extract of the lichen Parmelia perlata. Afri J Pharm and Pharmacol. 2008;2(6):106-109.
- Murugaesan S, Bhuvaneshwari S and Sivamurugan V. Evaluation of in vitro antidiabetic activity of red seaweed Portieria hornemannii (Silva) and Spyridia fusidormis (Wulfen).World journal of pharmaceutical sciences. 2016;4(6):415-419.
- Hussain M ,Masood S.R, farooq U , Bakhsh H ,Majeed A and Aziz A. In vitro Antimicrobial Potential of Lichen (Parmelia perlata) against different Pathogenic Microbes. International Journal of Pharma Sciences. 2014;4(4):666-670
- Jayanthi P and Lalitha P. Reducing power of the solvent extracts of Eichhornia crassipes (Mart.) Solms. International Journal of Pharmacy and Pharmaceutical sciences. 2011;3:126-128.
- P. Pineda M, Anguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a Phosphomolybdenum Complex: Specific application to the determination of Vitamin E. Anal. Biochem. 1999;269:337-341.
CrossRef - Priyanka B, Anitha K, Shirisha K, Janipasha S.K, Dipankar B, Rajesh K. Evaluation of antioxidant activity of ethanolic root extract of Albizia Lebbeck (L.) Benth .International Journal of Pharmaceutical and Applied sciences. 2013;3(2):93-101.
- Proksa B and Proksova A. Lichens metabolites Usnic acid and its biological activity. Farm .Obz. 1999;68:139–143.
- Rashmi S and Rajkumar H.G. Preliminary phytochemical screening of different solvent extracts of lichen from Kodagu district, Karnataka. Journal of Pharmacognosy and Phytochemistry. 2014;3(4):209-212.
- Shivanna R, Parizadeh H and Rajkumar H.G. Screening of Lichen extracts for in vitro antidiabetic activity using alpha amylase inhibitory assay. International Journal of Biological and Pharmaceutical Research. 2015;6(5):364-367.
- Ravindra C, Sanjay S,Kasture B and Kalaichelvan V.K. Phytochemical studies on the glycosides of leaf extracts of medicinally important plant Holoptera integrifolia (RoxB) planch using High performance thin layer chromatography. Asian Journal of Pharmaceutical and Clinical research. 2014;7( 4):197-200.
- Sharadanand R, Anup P, Hendre S. Total antioxidant capacity of fresh leaves of Kalanchoe pinnata. Journal of Pharmacognosy and Phytochemistry. 2014;2(5):32-35.
- Ruch R.J, Cheng S.J and Klaunig J.E. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. 1989;10:1003-1008.
- Yan R,luo N, yi B, zhao M, Liu Y,chen L. GC-Method for determination of Piperidine compounds and its application to the industrial process control of active pharmaceutical industries. International Research Journal of Pharmaceutical sciences. 2014;5(1):0017-0022.
- papitha R, Ravi L, Immanuel C.S. Phytochemical studies and GC-MS analysis of Spermadictyon suaveolens RoxB. International Journal of Pharmacy and Pharmaceutical sciences. 2017;9(3):143-149.
CrossRef - Reshmi S.K, Sathya E and Devi S.P. Isolation of Piperidine from Piper nigrum and its antiproliferative activity. African Journal of Pharmacy and Pharmacology. 2010;4(8):562-573.
- S. A Study of Invitro antioxidant activity of Parmotrema grayanum and its bioactive compounds. International Journal of Engineering sciences and Research technology. 2015;4(1):791-795.
- Iyo J.H, Tsujiyama M.T, Ashabul Md.I, Rajat S.B, Hitoshi A. Total phenolic content,antioxidative,anti-amylase,antiglucosidase and antihistamine release activities of Bangladeshi fruits. Food Science and Technology Research. 2008;14:261-268.
CrossRef - Apsara G.V, Dhananjaya K, Ravikumar K.R and Mallesha H . Phytochemical and Antibacterial properties of spices against food borne bacteria with special reference to Parmelia perlata. G.J.B.B. 2013;2(2):145-149.
- Viseshni R and Iyer P.R. Screening of Antidiabetic and Anticancer activity in Andrographis paniculata (Nilavembu).World Journal of Pharmacy and Pharmaceutical sciences. 2017;6( 8):1837-1849.

This work is licensed under a Creative Commons Attribution 4.0 International License.






