Manuscript accepted on : 17 August 2016
Published online on: --
Plagiarism Check: Yes
Dechlorination and Iron Extraction from Drinking Water by Fruit Pit Shell Activated Carbon
Usenkulova Sholpan Zhenisbekovna1, Marat Isakovich Satayev1, Samonin Vyacheslav Viktorovich2 and Aliya Merekeyeva Zhanybekovna1
1M. Auezov South Kazakhstan State University, Kazakhstan, Shymkent.
2St. Petersburg State Technological Institute (Technical University) Russia St. Petersburg.
Corresponding Author E-mail: maratsatayev@mail.ru
DOI : http://dx.doi.org/10.13005/bbra/2367
ABSTRACT: Fruit pit shell activated carbon was used as an adsorbent for dechlorination and iron extraction from drinking water. The adsorber design, allowing increase apparatus efficiency, increase use efficiency of the effective apparatus volume and treatment degree of the adsorbent’s adsorptive capacity in the layer, consistency of performance of the treated flow and apparatus compactness, was developed. The water flow velocity and treatment time influence on the residual chlorine absorbency was studied. Dependence of the chlorine adsorption value on the equilibrium concentration, as well as dependence of the iron adsorption value by the fruit pit shell activated carbon were determined. Adequate adsorption value by the chlorine equal to 0,02 mg/g and by the iron equal to 0,02 mg/g was attained. This method of the drinking water treatment using the activated carbon can be used in the treatment process of natural waters to remove the residual chlorine and formed chlorine compounds, as well as iron.
KEYWORDS: Active carbon; apricot shells; dechlorination; adsorption
Download this article as:| Copy the following to cite this article: Zhenisbekovna U. S, Satayev M. I, Viktorovich S. V, Zhanybekovna A. M. Dechlorination and Iron Extraction from Drinking Water by Fruit Pit Shell Activated Carbon . Biosci Biotech Res Asia 2016;13(4). |
| Copy the following to cite this URL: Zhenisbekovna U. S, Satayev M. I, Viktorovich S. V, Zhanybekovna A. M. Dechlorination and Iron Extraction from Drinking Water by Fruit Pit Shell Activated Carbon . Biosci Biotech Res Asia 2016;13(4). Available from: https://www.biotech-asia.org/?p=16903 |
Introduction
Fresh water delivery problem is actual for many countries, particularly for countries of Central Asian region [1]. In South Kazakhstan, the problem of quality drinking water delivery to the population alongside with natural water resource deficit is compounded by poor technical condition of water supply facilities. This results in deterioration of sanitation-and-epidemiological situation and incidence rate. Treated water must be suited to the standard requirements of the World Health Organization, U.S. Environmental Protection Agency, Council of the European Union and Sanitary rules and norms the Republic of Kazakhstan 2.1.4.1074-01. Such high purity degree of drinking water can be attained only using modern water treatment technologies. One of such effective methods for deep and fine water treatment is adsorptive technology.
Review [2] presents adsorptive properties of the fruit pit shell adsorbent in the water treatment. The adsorbent showed sure adsorptive properties in the adsorption of methylene blue, congo red, methyl rosaniline, rhodamine B, base red. The adsorption process strongly depends on pH medium.
[3] Studied cashew nutshell activated carbon activated by КОН and СО2 at a temperature of 1027 К. Increase in impregnation coefficient and activation time resulted in development of mesoporous structure with increase in mesopore volume ratio from 20-28 % and 27-45 %. Specific surface area by BET attained high values from 682 to 1026 m2/g. This adsorbent showed high adsorptive properties by Pb(II) – 99,61 % and Cd ions (II) 98,87 % at pН 6,5. Experimental data were estimated by Freundlich isotherms and Langmuir isotherm model.Extraction of toxic organic compounds (benzene, acetic aldehyde and acrylonitrile) using coconut shell activated carbons was studied [4]. Characteristic adsorption energies in micropores for the nutshells are higher than on the basis of resin based carbon. The effect of these different pore size characteristics on the adsorption kinetics, obtained by fitting the data to the linear driving force model, is that the resulting adsorption rate constants are higher across much of the relative pressure range (p/ps) studied for the resin based carbon compared to the nutshell material.
The paper presents Cr(VI) and Ni(II) adsorption results using the coconut shell activated carbon [5]. It is found that maximal adsorption of Cr (VI) is (94,5 %) at рН = 2,0, and for Ni(II) maximal extraction is (58,92 %) at рН = 9,0. The adsorption kinetic data of Cr(VI) and Ni(II) were found fitting well in pseudo second-order equation both in single and binary system (r2>0.99) and intraparticle diffusion was the rate controlling step. The negative ΔG° and the positive ΔH° indicated the spontaneous and endothermic nature of the adsorption process. The extended Langmuir isotherm model fitted well with the competitive adsorption data of Cr(VI) and Ni(II). For the desorption experiments, EDTA showed the maximum desorption efficiency of 69 % for Cr(VI) and 81 % for Ni(II).
The adsorption method is considered to be the cheapest and the most effective to remove fluoride from the drinking water [6]. Investigations to determine efficiency of the fluorides’ removal from the water solution using nutshell activated carbon were conducted. In the initial fluoride concentration (2-100 mg/l) and solution temperature (25-45 °С) the fluoride removal attained up to 46 %. The obtained adsorption data were analyzed using the Langmuir and Freundlich isotherm models showing that fluoride adsorption onto nutshell-based activated carbon fits the Freundlich model well.
Adsorptive properties of activated carbon based on products from Lantana camara stem were studied [7]. The agricultural material (Lantana camara stem) was carbonized at 300˚C for 2 h, ground and steam-activated. Methylene blue uptake increased with increase in pH, contact time and attained the balance after 60 minutes. Dye removal increased when the dose was increased from 0,5-2,0 g/l, at different dye concentrations (50 – 200 mg/l). This study suggests that adsorbent prepared from Lantana camara stem can be used effectively for the adsorption of methylene blue in wastewater.
The paper [8] presents review about the role of date-palm as the adsorbents in removal of undesirable materials, such as acids and basic dyes, heavy metals and phenol compounds. It was shown that date-palm based adsorbents are the most promising sorbents to remove undesirable materials.
Purpose of the study [9] was to estimate application of granulated activated carbon obtained from shells of almond, English walnut, pecanin «Envirofilter» water filtration systems and determine its efficiency in removal of chlorination by-products, namely trihalomethanes bromodichloromethane, bromoform and dibromochloromethane. Eight various «Envirofilter» were made using the activated carbons obtained from the shells of almond, walnut, pecan activated by acid or steam. «Envirofilter» may be considered as a substitution of available commercial filtration systems.
The paper [10] studied adsorption of Rhodamine В (RB) dye from water solutions using walnut shell activated carbon. Maximal adsorption was attained at pH equal to 3,0. Positive standard enthalpy (H°) values were obtained indicating that the RB adsorption process is endothermic as well as ΔG° and ΔS° values showed that adsorption process is spontaneous with an increased randomness at the solid-liquid interface. Desorption studies were carried out to explore the feasibility of regenerating the used walnut shells and it was found that 97,71 – 99,17 % of the retained RB was recovered with 0,1 mol/l NaOH solution. The walnut shells were also successfully used to remove RB from industrial effluents.
The paper carried out investigations to remove nitrates from water by activated carbon and clinoptilolite, the natural zeolite [11]. Adsorption of the nitrates was carried out at various contact time, temperature, pH, dose of the adsorbent and initial nitrate concentration. For the activated carbon in amount of 4 g, 60 minutes contact time, water temperature of 20°C, рН = 6,5 and initial concentration of 100 mg/l, the nitrate removal efficiency from 60 ml of water was 62,61 %. From the adsorption kinetics, it may be concluded that it is expedient to adsorb the nitrate from water using the activated carbon and clinoptilolite, the natural zeolite.
Investigations of wild endemic almond based adsorbent were carried out: Amygdalus scoparia shellwas estimated to adsorb Pb2+, Ni2+, Cr6+ and Cr3+ heavy metal ions from water solution [12]. The adsorptive capacity was studied taking into account effect of various parameters, such as contact time, initial concentration, pH, temperature, doses and particle size. Results of this investigation showed that Amygdalus scoparia shellmay adsorb high level of Plumbum, Chrome(VI) and (III) during short period of time, and their intake rate to a great extent depends on their concentration in a contaminated water. At the almond shell contact time of 48 hours, it was found that the removal efficiency for Cr(VI) was 87,1 %, Cr(III) – 89,8 %, PB – 84,4 % and Ni – 48,1 %.
The paper studied adsorptive properties of two wild endemic almonds: Amygdalus lycioides and Amygdalus wendelboi shells when extracting Ni2+, Cr6+ and Cr3+ heavy metal ions from water solution [13]. At the contact time of 48 hours, it was found by both adsorbents from almond shellthat the removal efficiency for Cr(VI) was 83,6 %, Cr(III) – 94,1 %, Ni – 94,1 %.
In this connection, it is very actual to develop and introduce promising, low-waste and high-efficiency adsorption cleaning of surface waters, the source of drinking water supply.
As distinguished from available apparatus-technological solutions, the adsorption technology provides drawing thoroughly purified water from municipal water supply network, as well as from drinking reservoirs.
Material and methods
Torlan water storage, the source of drinking water on the territory of South Kazakhstan in Suzak district, Sholak-Korgan village, with population of more than 20 000 people, was chosen as the research subject. Personnel of M. Auezov South Kazakhstan State University jointly with personnel of «Sholak-Korgan-Su» public utility service carried out sanitary assessment of the drinking water chemical pollution and its impact risk on the population’s health. There is Standard for drinking water – GOST 2874-73 [14] in Kazakhstan. It covers drinking water supplied by centralized utility and drinking water supply systems, as well as by centralized water supply facilities, delivering water for both utility and drinking and technical purposes and sets sanitary requirements and control for the drinking water quality.
Table 1 presents qualitative indicators of Torlan water storage influent water.
Table 1: Qualitative indicators of Torlan water storage influent water
| No. | Indicators | Unit of measure | Base values of Sholak-Korgan-Su |
| 1 | Temperature | Degrees | 15 |
| 2 | Odor at a temperature of 200С, no more than | Grade | 3,0 |
| 3 | Odor at a temperature of 600С, no more than | Grade | 3,0 |
| 4 | Taste and after-taste at a temperature of 200С, no more than | Grade | 3,0 |
| 5 | Color, no more than | Degrees | 80 |
| 6 | Turbidity, no more than | mg/dm3 | 7,0 |
| 7 | рН | рН unit | 5,8 |
| 8 | Total hardness, no more than | meq/dm3 | 11,0 |
| 9 | Dry residual, no more than | mg/dm3 | 1500 |
| 10 | Fluorides | mg/dm3 | 5,0 |
| 11 | Iron, no more than | mg/dm3 | 1,0 |
| 12 | Residual chlorine | mg/dm3 | – |
| 13 | Pb | mg/dm3 | <0,003 |
| 14 | Total alpha activity | Bq/dm3 | <0,1 |
| 15 | Totalbeta activity | Bq/dm3 | <0,1 |
| 16 | Sulfates, no more than | mg/dm3 | 600 |
| 17 | Chlorides, no more than | mg/dm3 | 510 |
Apricot pit shell activated carbon, carbonized in Nitrogen at a temperature of 700 оС and activated in the flow of СO2 and H2O in the mass ratio 85:15 at a temperature of 800 °С was used at the adsorbent. Table 2 presents characteristics of the activated carbon. The apricot pit shell is the heavy tonnage wastes of canning plants for production of fruit cannery products and oil-and-fat plants for processing of pit raw material. Available volume of the raw material in the Central-Asian region, its low price and transportability, good enough adsorptive and physical-chemical properties, as well as effective methods for regulation of the porous structure make possible and economically feasible their use as the adsorbents for water purification.
Table 2: Characteristics of the activated carbon
| Moisture content, % | Total porousness, cm3/g | Mesopore volume, cm3/g | Micropore volume, cm3/g | Macropore volume, cm3/g | Brightening ability by methylene blue, % | Ash content, % |
| Offered carbon | ||||||
| 1 | 1,00 | 0,15 | 0,65 | 0,20 | 200 | <6 |
The adsorption plant presented on Figures 1, 2 and 3 was used with a view to study the adsorption water cleaning.
Disadvantages of available apparatus are their low operating efficiency, under exploitation of the adsorbent capacity, low utilization of the apparatus’ useful capacity and treatment degree of the adsorbent’s adsorptive capacity in the layer.
The task was to develop the adsorber design, allowing increase efficiency of the apparatus, increase the apparatus’ useful capacity utilization and treatment degree of the adsorbent’s adsorptive capacity in the layer, stability of the purified flow quality and compactness of the apparatus.
The assigned task is solved by the fact that the adsorber, containing body, contact chamber of the adsorbent with the purified flow, connections for supply and output of the flow, connections for supply and output of reclaiming agent, fitting, joining connections for supply and output of the reclaiming agent, doors for feed and discharge of the adsorbent, according to the invention, is additionally equipped by vertical contact chamber of the adsorbent with the flow, set along the apparatus axis, with round section and joint with the connection for supply and output of the reclaiming agent, at that the lower side of the inner chamber is solid.
Nature of the invention is explained by drawings, where Figure 1 shows cut of the adsorber at the section, Figure 2 shows cut of the adsorber at the section A-A, Figure 3 – pattern of water movement in the adsorber.
The adsorber contains body 1, outer 2 and inner 3 contact chambers of the adsorbent with the purified flow, connection 4, coarse filter 5 and crane 6 for initial supply of the flow, connection 7 and crane 8 for output of the purified flow, connection 9 and crane for feed of the reclaiming agent, connection 10 and crane 11 for discharge of the reclaiming agent at regeneration, doors 9 for feed and discharge of the adsorbent.
The adsorber operates as follows.
The flow, subjected to be purified through the connection 4, coarse filter 5 and crane 6, enters into the inner part of the adsorber body 1, passes in horizontal direction through the ring carbon layer, which is in the inner contact chamber 5 of the adsorbent with the purified flow. Further, the purified flow is supplied to the upper part of the inner contact chamber 3 of the adsorbent with the purified flow, where the flow is additionally filtered through the adsorbent layer and discharged from the apparatus through the connection 7 and crane 8.
When reducing degree of the flow purification by the adsorbent, as evidenced by increase in concentration of the intake substance in the purified flow, the filtering load (the adsorbent layer) is regenerated. To this end, with the help of the cranes 6, 8, connections 4, 7, the initial flow supply is cut off, the reclaiming agent is loaded through the connection 9 and crane 10 and supplied to the contact chambers 3 and 2. The intake substance, separated from the adsorbent, in the mixture with the reclaiming agent is discharged through the connection 11 and crane 12. The feed and discharge of the adsorbent is made through the doors 9.
By installation of the additional coarse filter and simplified inner contact chamber of the adsorbent with the purified flow, the apparatus efficiency increases, use efficiency of the effective apparatus volume and treatment degree of the adsorbent’s adsorptive capacity in the layer, consistency of performance of the purified flow in the maximal apparatus compactness increase.
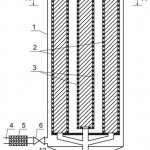 |
Figure 1: Cut of the adsorber at the section
|
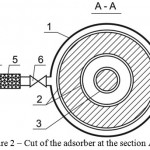 |
Figure 2: Cut of the adsorber at the section A-A
|
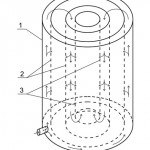 |
Figure 3: Pattern of water movement in the adsorber
|
Results and discussion
Various chlorination modes can be used depending on the source natural water quality [15, 16]. Single chlorination with introduction of chlorine and several chlorine-containing agents into water before clean-water reservoir is usually used for those waters, which contain comparatively many harmful substances and bacteria. Minimal chlorine doses within the limits of 0,5 – 2 mg/l, as a rule, are used in this case. Double chlorination is often used when clarifying strongly colored waters, as well as waters, rich by harmful substances, toxic impurities and bacteria.
Water disinfection by chlorine may result in hazardous adverse effect on halogen-derivative organisms. Dechlorination of water may be carried out by physical method – by chlorine uptake by the adsorbent. Advantage of the dechlorination method is absence of need to add some additional reagents.
Influence of the water flow velocity and treatment time on the residual chlorine absorbency by the activated carbon is shown on Figures 4 and 5. It is seen that the optimal mode factors are treatment time of the process – 50 minutes; water flow velocity – 0,003 m/s.
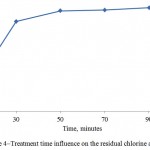 |
Figure 4: Treatment time influence on the residual chlorine absorbency
|
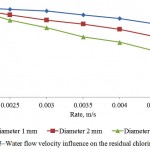 |
Figure 5: Water flow velocity influence on the residual chlorine absorbency
|
Investigations on the adsorptive extraction of the residual chlorine, where it is seen from Figure 6 that sufficient adsorption value equals to 0,02 mg/g during 1 hour of the adsorption, were carried out to study water treatment from the residual chlorine, contained in the surface waters.
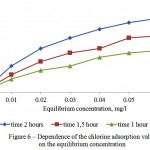 |
Figure 6: Dependence of the chlorine adsorption value on the equilibrium concentration
|
Table 3 presents content of the residual chlorine and derivation degree in percent from the initial content. It is seen from the table that the chlorine derivation degree is high enough for application of this activated carbon and adsorptive apparatus.
Table 3: Content of the residual chlorine and derivation degree in percent from the initial content
| Adsorbent
|
Residual chlorine concentration | Derivation degree of the residual chlorine from the initial, % | |
| In the source water, mg/l | In the purified water, mg/l | ||
| Activated carbon
|
0,6 | 0,001 | 99,0 |
Treatment degree efficiency when using the activated carbon in the residual chlorine extraction from water attains 99 %. This extraction method of the residual chlorine using the activated carbon can be used in purification of natural waters to remove the residual chlorine, as well as formed chlorine compounds.
Water may contain several types of iron. In the surface waters, iron as an impurity is mainly contained in organic complexes (humates), and also forms colloidal and finely dispersed suspensions. Currently, it is established that continuous consumption of water with increased iron content (more than 0,3 mg/l) raises risk of infarcts and negatively influences on reproductive function.
Figures 7 and 8 show sufficient adsorptive capacity of activated carbons by iron. At equilibrium concentration in 0,6 mg/l, the adsorption value attains 0,7 mg/g of the adsorbent. Good adsorptive properties are conditioned by developed specific surface and microporosity of the adsorbent.
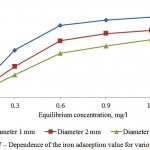 |
Figure 7: Dependence of the iron adsorption value for various adsorbents
|
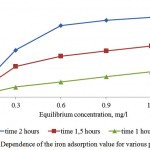 |
Figure 8: Dependence of the iron adsorption value for various porous structures
|
The offered apparatus completely substitutes apparatus of chlorination, ozonizing, high-temperature water processing, ion-exchange purification. The deep purification is carried out without additional introduction of reagents, it is non-waste and simple in use.
Analyzing the data, it may be concluded that after the adsorptive purification the drinking water quality by its organoleptic, physical-chemical and microbiological indicators meets the requirements of Sanitary rules and norms 3.01.067-97 «Drinking water».
Conclusion
Design of the adsorber with stationary layer of the adsorbent, allowing increase the apparatus efficiency, increase use efficiency of the effective apparatus volume and treatment degree of the adsorbent’s adsorptive capacity in the layer, consistency of performance of the treated flow in the maximal apparatus compactness, reduce formation of nonmoving and unused zones was developed.
Influence of the water flow velocity and treatment time on the residual chlorine absorbency by the activated carbon, where the optimal mode factors are treatment time of the process – 50 minutes; water flow velocity – 0,003 m/s, was determined. Dependences of the iron adsorption value for the activated carbon based on the fruit pit shells were determined.
Carried out experimental investigations on the adsorption of the residual chlorine and iron by the activated carbon, thus, established the possibility to increase the adsorption in the sufficient degree to obtain water with the quality meeting the normative standards.
References
- Musabekov, A.A., Satayev, M.I., Altynbekov, F.Ye. Otsenka kachestva vody po kompleksnoy ekologicheskoy klassifikatsii kachestva poverkhnostnykh vod Yuzhnogo Kazakhstana. Sbornik trudov VIII mezhdunarodnoy nauchno-prakticheskoy konferentsii «Issledovaniye, razrabotka i primeneniye vysokikh tekhnologii v promyshlennosti», Sankt-Peterburg, 2009; 1: 295-299.
- Pankaj Sharma, Harleen Kaur, Monika Sharma, Vishal Sahore. A review on applicability of naturally available adsorbents for the removal of hazardous dyes from aqueous waste. Environ Monit Assess, 2011; 183:151–195
CrossRef - Tangjuank, S., Insuk, N., Tontrakoon, J., Udeye, V. Adsorption of Lead(II) and Cadmium(II) ions from aqueous solutions by adsorption on activated carbon prepared from cashew nut shells. International Journal of Chemical, Molecular, Nuclear, Materials and Metallurgical Engineering, 2009; 3(4): 221-227.
- Peter Branton, Robert H. Bradley. Effects of active carbon pore size distributions on adsorption of toxic organic compounds. Adsorption, 2011; 17: 293–301.
CrossRef - Yunhai Wu, Palizhati Yilihan, Julin Cao, Yanping Jin. Competitive adsorption of Cr (VI) and Ni(II) on to coconut shell activated carbon in single and binary systems. Water Air Soil Pollut, 2013; 224:1662.
CrossRef - Ergović Ravančić M., Habuda-Stanić M. Fluoride removal from water using nutshell-based adsorbent. Proceedings of the 14th International Conference on Environmental Science and Technology (CEST2015_00464), Rhodes, Greece, 2015
- Omotayo Sarafadeen Amuda, Akeem Olusegun Olayiwola, Abass Olanrewaju Alade, Abolaji Grace Farombi, Segun Akanmu Adebisi. Methylene blue from aqueous solution using steam-activated carbon produced from lantana camara stem. Journal of Environmental Protection, 2014; 5: 1352-1363.
CrossRef - Tanweer Ahmad, Mohammad Danish, Mohammad Rafatullah, Arniza Ghazali, Othman Sulaiman, Rokiah Hashim, Mohamad Nasir, Mohamad Ibrahim. The use of date palm as a potential adsorbent for wastewater treatment: a review. Environ SciPollut Res, 2012; 19:1464–1484.
CrossRef - Mohamed Ahmedna, Wayne E Marshall, Abdo A Husseiny, Ipek Goktepe, Ramu M Rao. The use of nutshell carbons in drinking water filters for removal of chlorination by-products. J ChemTechnolBiotechnol, 2004;79:1092–1097.
CrossRef - Jasmin Shah, M. Rasul Jan, Altaul Haq. Vounas Khan. Removal of Rhodamine В from aqueous solutions and wastewater by walnut shells: kinetics, equilibrium and thermodynamics studies. Front. Сhem. Sci. Eng., 2013; 7(4): 428- 436.
- Morteza Kashefi As, Amir Hesam Hasani, Ehsan Naserkhaki. Evaluation of nitrate removal from water using activated carbon and clinoptilolite by adsorption method. Biosciences biotechnology research asia, 2016; 13(2): 1045-1054.
CrossRef - Parisa Ziarati, Fatemehsadat Mir Mohammad-Makki, Maryam Moslehishad. Novel adsorption method for contaminated water by wild endemic almond: Amygdalus scoparia. Biosciences biotechnology research asia, 2016; 13(1); 147-153.
- Parisa Ziarati, Armaghan Kermanshah, Maryam Moslehishad. Adsorption heavy metal from contaminated water by modified shell of wild endemic almonds: Amygdalus lycioides and Amygdalus wendelboi. Biosciences biotechnology research asia, 2015; 12(3): 2451-2457.
CrossRef - GOST 2874-82. Voda pit’yevaya. Gigiyenicheskiye trebovaniya i kontrol’ za kachestvom.
- Kastal’skiy, A.A. Podgotovka vody dlya pit’yevogo i promyshlennogo vodosnabzheniya. Moscow, Vysshaya shkola, 1962: 555 p.
- Nikoladze, G.N. Tekhnologiya ochistki prirodnykh vod. Moscow, Vysshaya shkola, 1987: 479 p.

This work is licensed under a Creative Commons Attribution 4.0 International License.





