Manuscript accepted on : 20 September 2016
Published online on: --
Plagiarism Check: Yes
Roy Amit and Prasad Pushpa
Columbia Institute of Pharmacy, Tekari, Raipur (CG)-493111, India.
Corresponding Author E-mail: pushpaprasad81@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2372
ABSTRACT: The diabetes can be controlled by inhibiting major carbohydrate hydrolyzing enzymes lead to reduce postprandial hyperglycemia. The aim of the study was to evaluate the antidiabetic activity of oven dried extract (ODE) and lyophilized extract (LE) of Calocybe indica using enzyme inhibitory assays. The ODE and LE were tested against α-amylase, α-glucosidase, and dipeptidyl peptidase-IV (DPP-IV). The ODE demonstrated 50% inhibitory activity for α-amylase, α-glucosidase and DPP-IV enzyme at 62.18 μg/ml, 47.77 μg/ml and 91.84 μg/ml, respectively. The LE revealed 50% inhibitory activity for α-amylase, α-glucosidase and DPP-IV enzyme at 38.11 μg/ml, 28.09 μg/ml and 60.91 μg/ml, respectively. Furthermore, the IC50 values of LE are nearer to IC50 values of standard drug Acarbose. The lyophilized extract possessed higher enzymatic inhibitory activity compared to oven dried extract due to restoring efficiency of secondary metabolites.
KEYWORDS: Calocybe indica; α-amylase; α-glucosidase; dipeptidyl peptidase-IV
Download this article as:| Copy the following to cite this article: Amit R, Pushpa P. Assessment of Mechanism of Action of Antidiabetic Activity of Calocybe indica by Enzyme Inhibitory Activity. Biosci Biotech Res Asia 2016;13(4). |
| Copy the following to cite this URL: Amit R, Pushpa P. Assessment of Mechanism of Action of Antidiabetic Activity of Calocybe indica by Enzyme Inhibitory Activity. Biosci Biotech Res Asia 2016;13(4). Available from: https://www.biotech-asia.org/?p=16740 |
Introduction
Diabetes is allied with disorders of carbohydrate, fat and protein metabolism. It is distinguished by irrelevant hyperglycemia produced by insufficiency of insulin at the cellular level1. It is reported diabetic patient are increasing every year globally2, and inferring that more than 400 million people of the worldwide will be effected from hyperglycemia by 20303. The occurrence rate of diabetes in India is 1-5%4. Severe diabetes patient are associated with various disease namely neuropathy, hyperlipemia, nephropathy and retinopathy. Hence it is essential to control the diabetes and various types of synthetic drugs are available in market. The demand of herbal drug for the treatment of diabetes is enhanced due to the side effects associated with the use of insulin and oral hypoglycemic agents2. Presently worldwide practices herbal drugs for the ailment of diabetes. The World Health Organization suggested using of herbal medicines where the conventional treatment of diabetes is not satisfactory3.
The medicinal plants exhibited an antidiabetic property due to its chemical constituents present inside the plants. The secondary metabolites of plants have an ability to enhance glucose transport and metabolism in muscle and/or to stimulate insulin secretion. The possible mechanism for antidiabetic activity of natural products is due to its action on carbohydrate binding regions of α-glucosidase enzyme and α- amylase. Further it stimulates the hydrolysis of the internal α-1, 4 glucosidic linkages in starch and other allied polysaccharides for the prevention of postprandial hyperglycemia. The above mentioned enzyme hydrolyzes the dietary starch into maltose which then breaks down to glucose prior to absorption. The phytoconstituents such as flavonoids and polyphenol components play a chief role to delay digestion and absorption of carbohydrates lowering the postprandial glucose levels by inhibiting α-glucosidase and α- amylase enzyme6,7.
Calocybe indica belongs to family Tricholomataceae, is rich in protein, flavonoids, lipid, fiber, carbohydrate and vitamin. Calocybe indica are used as food supplement in diet and also used in the form of medicines due to presence of phenolic compounds, terpenes, polyketides, sterols, ergosterol, flavanoids and steroids. The various researchers documented that Calocybe indica are efficient in lessening the total plasma cholesterol and triglyceride level. The polyphenol and flavonoids present in Calocybe indica impede oxidative damage by free radical and reactive oxygen species. Consequently, it restrains the development of various diseases like ageing, carcinogenesis, obesity and diabetes. Additionally, the tribal people claim the antidiabetic use of Calocybe indica8-10. Hence we planned to investigate the antidiabetic activity of Calocybe indica by in vitro enzyme inhibitory activity.
Materials and Methods
Plant material
The Calocybe indica spawn was collected from Mushroom Research Centre, Plant Pathology Department, Indira Gandhi Krishi Vishwavidyalaya, Raipur, Chhattisgarh. Further it was cultivated in Columbia Mushroom Centre, Raipur, Chhattisgarh. The cultivated Calocybe indica was collected and cleaning was performed to remove soil and straw from the mushroom. Calocybe indica was dried at room temperature to remove moisture from it. Immediately after drying Calocybe indica was subjected for grinding to get coarse powder for further processes.
Preparation of oven dried and lyophilized extracts
70% ethanol was used for the maceration of Calocybe indica. The extraction process was continuing for 7 days, solution was shaken continuously with orbital shaker for six hours every day. After 7 days extract was filtered and the resultant extract was divided into two parts: one part for oven dried by maintaining temperature at 60 °C and extract was kept in air tight container for further use. It was represented by oven dried extract (ODE).
Second part of extract was subjected to lyophilization and extract was stored at -20 °C until use. It was represented by lyophilized extract (LE).
Enzyme inhibitory activities by α-amylase
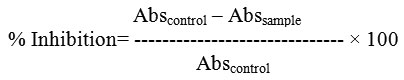
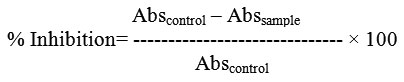
The assay mixture containing 200 μl of 0.02 M sodium phosphate buffer, 20 μl of enzyme and ODE, LE and Acarbose at different concentration (20-100 μg/ml), separately was incubated for 10 minutes at room temperature followed by addition of 200 μl of starch in all test tubes. The reaction was terminated with the addition of 400 μl DNS reagent and placed in boiling water bath for 5 minutes, cooled and diluted with 15 ml of distilled water and absorbance was measured at 540 nm. The control samples were prepared without any test sample. The % inhibition was calculated according to the formula11.

Enzyme inhibitory activities by α-glucosidase
The α-glucosidase inhibitory activity was determined by measuring the release of 4-nitrophenol from p-nitrophenyl α-D glucopyranoside. The assay mixtures for these experiments contained 0.3 ml of 10 mM p-nitrophenyl α-D-glucopyranoside, 1.0 ml of potassium phosphate (0.1M, pH: 6.8), 0.2 ml of enzyme solution and 0.2 ml of inhibitor ODE, LE and Acarbose at different concentration (20-100 μg/ml), separately, all in a final volume of 1.7 ml. Following an incubation time of 30 min at 37º C, the reaction was terminated by the addition of 2.0 ml of 100 mM sodium carbonate. The liberated p-nitrophenol was determined at 400 nm using spectrophotometer12. The % inhibition rates were calculated using the formula,
Enzyme inhibitory activities by Dipeptidyl peptidase IV (DPP-IV)
DPP-IV-inhibitory activity (IC50) was determined using a modified method of Konrad (2014). DPP-IV from goat intestine was resuspended in 0.1 M/L Tris–HCl buffer, pH 8.0. The ODE and LE (25μL) was pre-incubated with the equal volume of the substrate Gly- Pro-p-nitroanilide (1.6 mM) at 37 ºC for 10 min. Afterwards, 50μL of DPP-IV (0.01 U/mL, in 0.1 M/L Tris–HCl buffer, pH 8.0) was added and the mixture was incubated at 37º C for 60 min. The reaction was stopped by the addition of 100μL of 1 M/L sodium acetate buffer, pH 4.0. The released p-nitroanilide as a hydrolysis product was measured at 405 nm13.
Statistical analysis
The values of results were expressed as mean value ± S.E.M. the variation in a set of data has been estimated by performing was analysis of variance (ANOVA). Individual comparison of group means values were done by using Graph pad prism 7.01.
Results
Inhibitory activity of ODE and LE on α-amylase enzyme
The finding of inhibitory activity of ODE and LE are represented in the table 1. The IC50 value of ODE and LE were 62.18 μg/ml and 38.11 μg/ml, respectively for on α-amylase enzyme (Fig 1 and Fig 2). The standard drug Acarbose exhibited 50% inhibition on α-amylase enzyme at 20.24 μg/ml (Fig 3). The LE demonstrated higher inhibitory property for α-amylase enzyme compared to ODE.
Table 1: Percentage inhibition of wheat alpha-amylase by ODE, LE and Acarbose
| Concentration
(μg/ml) |
% inhibition by ODE | IC50 (μg/ml)
ODE |
% inhibition by
LE |
IC50 (μg/ml)
LE |
% inhibition by
Acarbose |
IC50 (μg/ml)
Acarbose |
| 20 | 18.36±0.43 | 62.18 | 28.18±0.92 | 38.11 | 49.35±0.31 | 20.24 |
| 40 | 30.24±0.68 | 51.95±0.41 | 75.52±0.52 | |||
| 60 | 45.16±0.37 | 79.12±0.83 | 93.81±0.94 | |||
| 80 | 68.27±0.69 | 91.47±0.64 | 116.32±0.47 | |||
| 100 | 79.43±0.28 | 105.62±0.73 | 138.73±0.18 |
Values are mean ± SEM of three determinations
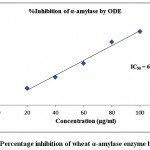 |
Figure 1: Percentage inhibition of wheat α-amylase enzyme by ODE
|
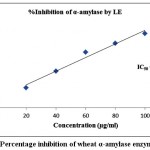 |
Figure 2: Percentage inhibition of wheat α-amylase enzyme by LE
|
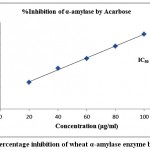 |
Figure 3: Percentage inhibition of wheat α-amylase enzyme by Acarbose
|
Inhibitory activity of ODE and LE on α-glucosidase enzyme
Table 2 demonstrated the outcomes of inhibitory activity of ODE and LE for α-glucosidase enzyme. The ODE and LE demonstrated 50% inhibitory activity for α-glucosidase enzyme at 47.77 μg/ml and 28.09 μg/ml, respectively (Fig 4 and Fig 5). The standard drug Acarbose exhibited 50% inhibition on α-glucosidase enzyme at 26.21 μg/ml (Fig 6). The findings indicate that LE produces higher inhibitory property for α-glucosidase enzyme compared to ODE. In addition, the ODE and LE produces higher inhibitory activity for α-glucosidase enzyme compared to α-amylase enzyme.
Table 2: Percentage inhibition of α-glucosidase enzyme by ODE, LE and Acarbose
| Concentration
(μg/ml) |
% inhibition by ODE | IC50 (μg/ml)
ODE |
% inhibition by
LE |
IC50 (μg/ml)
LE |
% inhibition by
Acarbose |
IC50 (μg/ml)
Acarbose |
| 20 | 23.51±0.85 | 47.77 | 38.24±0.51 | 28.09 | 41.32±0.29 | 26.21 |
| 40 | 41.72±0.63 | 63.42±0.63 | 68.53±0.38 | |||
| 60 | 63.35±0.54 | 85.18±0.49 | 86.39±0.42 | |||
| 80 | 81.15±0.78 | 105.37±0.63 | 112.48±0.93 | |||
| 100 | 97.62±0.39 | 124.49±0.57 | 132.61±0.71 |
Values are mean ± SEM of three determinations
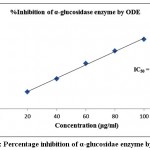 |
Figure 4: Percentage inhibition of α-glucosidae enzyme by ODE
|
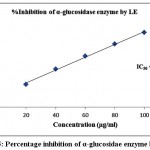 |
Figure 5: Percentage inhibition of α-glucosidae enzyme by LE
|
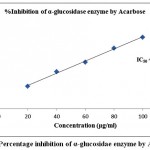 |
Figure 6: Percentage inhibition of α-glucosidae enzyme by Acarbose
|
Inhibitory activity of ODE and LE on DPP-IV enzyme
Table 3 exhibited the results of inhibitory activity of ODE and LE for DPP-IV enzyme. The ODE and LE revealed 50% inhibitory activity for α-glucosidase enzyme at 91.84 μg/ml and 60.91 μg/ml, respectively (Fig 7 and Fig 8). The standard drug Acarbose displayed 50% inhibition on DPP-IV enzyme at 34.24 μg/ml (Fig 9). The findings indicate that LE produces higher inhibitory property for DPP-IV enzyme compared to ODE. Consequently, the ODE and LE produces lower inhibitory activity for DPP-IV enzyme compared to α-glucosidase enzyme and α-amylase enzyme.
Table 3: Percentage inhibition of DPP-IV enzyme inhibition by ODE, LE and Acarbose
| Concentration
(μg/ml) |
% inhibition by ODE | IC50 (μg/ml)
ODE |
% inhibition by
LE |
IC50 (μg/ml)
LE |
% inhibition by
Acarbose |
IC50 (μg/ml)
Acarbose |
| 20 | 12.83±0.53 | 91.84 | 19.42±0.38 | 60.91 | 35.47±0.84 | 34.24 |
| 40 | 19.35±0.28 | 29.53±0.61 | 58.68±0.53 | |||
| 60 | 30.46±0.83 | 43.61±0.45 | 72.15±0.76 | |||
| 80 | 42.71±0.71 | 68.34±0.68 | 96.54±0.24 | |||
| 100 | 56.51±0.37 | 85.19±0.91 | 118.62±0.39 |
Values are mean ± SEM of three determinations
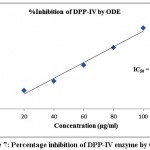 |
Figure 7: Percentage inhibition of DPP-IV enzyme by ODE
|
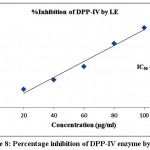 |
Figure 8: Percentage inhibition of DPP-IV enzyme by LE
|
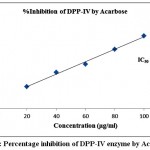 |
Figure 9: Percentage inhibition of DPP-IV enzyme by Acarbose
|
Discussion
Diabetes patients are identified by abnormal postprandial increase of blood glucose level. The α-amylase and α-glucosidase enzyme play chief role to enhance the glucose level in blood by catalyzing the release of α-glucose from the non-reducing end of the substrate1. The increment of blood sugar following carbohydrates meal can be reduced by inhibiting the α-amylase and α-glucosidase enzyme14. These enzymes are present in epithelium of the small intestine, and enzyme enable the absorption of glucose by the small intestine by catalyzing the hydrolytic cleavage of oligosaccharides into absorbable monosaccharides15. Consequently, on inhibiting the α-amylase and α-glucosidase enzyme in the small intestine, it decrease conversion rate of hydrolytic cleavage of oligosaccharide and the process of carbohydrate digestion spreads to the lower part of small intestine. This digestion process of carbohydrate delays the total absorption rate of glucose and declines the postprandial blood glucose peak in diabetic patients16-18.
The findings of present study, ODE and LE demonstrated inhibition against both α-amylase and α-glucosidase enzyme. The results implies that the extract of Calocybe indica were potent inhibitors of α-amylase and α-glucosidase enzyme. The lyophilized extract revealed higher inhibitory property compared to oven dried extract of Calocybe indica. Moreover, the IC50 values of LE are nearer to IC50 values of Acarbose and therefore can be potentially useful as an effective therapy for postprandial hyperglycemia with minimal side effects. The study supports the data of Picot et al19. stated natural α-glucosidase inhibitors from plants to have strong inhibition towards the activity of the enzyme compared to Acarbose.
The glucagon like peptide-1 (GLP-1), a potent insulinotropic peptide and capable to control the blood glucose level in Type 2 diabetes. GLP-1 can stimulate the release of insulin or suppressed the release of glucagon. DPP-IV enzyme inhibits the stimulation of GLP-1 and enhances the blood sugar level20. The DPP-IV inhibition is an approach to extend the circulating half-life of GLP-1, thus making DPP-IV inhibitors a promising target for the treatment of type 2 diabetes. The outcomes of study exhibited inhibitory effect of ODE and LE on DPP-IV enzyme, and effective approach to treat type 2 diabetes mellitus by potentiating insulin secretion.
The medicinal property of herbal drugs are depends upon the nature and quality of phytoconstituents present in herbs. It has been reported that the mostly phytoconstituents are thermolabile and sensitive to oven temperatures. The drying process of extract degrades the quality and efficacy of phytoconstituents. The freeze drying method is good option to overcome these problems. The present study revealed that lyophilized extract were more potent compared oven dry extract of Calocybe indica, and it indicates the restoring efficiency of chemical compounds in lyophilize extracts. Hence the findings of present study exhibited that the oven dried and lyophilized extract of Calocybe indica incorporating potent α-glucosidase, α-amylase and DPP-IV inhibitors, and were effective for suppressing postprandial hyperglycemia. The Calocybe indica should be given as food supplement for management of diabetes.
Conclusion
The present study demonstrated that the oven dried and lyophilized extract of Calocybe indica produces α-amylase, α-glucosidase, and DPP-IV inhibitory activity. It is predicted that oven dried and lyophilized extract of Calocybe indica suppress the glycemic response in diabetic conditions. The lyophilized extract possessed higher enzymatic inhibitory activity compared to oven dried extract due to restoring efficiency of chemical compounds. Further in vivo antidiabetic and cell line study are require to confirm the exact mechanism of Calocybe indica.
References
- Sidhu, A.K., Wani, S.J., Tamboli, P.S., Patil, S.N. In Vitro Evaluation of Anti-Diabetic Activity of Leaf and Callus Extracts of Costus pictus. International Journal of Science and Research, 2014; 3(4): 1622-1625.
- Samyal, M.L., Ahuja, A., Ahmed, Z. Estimation of Antihyperglycemic and Antihyperlipidemic Activity of Isolated Fractions from Ficus glomerata Bark Extract in Streptozotocin-Induced Diabetic Rats. UK Journal of Pharmaceutical and Biosciences, 2014; 2(5): 43-48.
CrossRef - Samyal, M.L., Ahuja, A., Ahmed, Z. Evaluation of Antidiabetic Activity of Isolated Compound from Ougeinia oojeinensis Bark Extract in Diabetic Rats. UK Journal of Pharmaceutical and Biosciences, 2014; 2(5): 27-33.
CrossRef - Sen, P., Sahu, K., Prasad, P., Chandrakr, S., Sahu, R.K., Roy, A. Approach to Phytochemistry and Mechaniasm of Action of Plants having Antidiabetic Activity. UK Journal of Pharmaceutical and Biosciences, 2016; 4(1): 82-120.
CrossRef - Liu, C., Song, J., Teng, M , et al. Antidiabetic and Antinephritic Activities of Aqueous Extract ofCordyceps militaris Fruit Body in Diet-Streptozotocin-Induced Diabetic Sprague Dawley Rats. Oxidative Medicine and Cellular Longevity, 2016; (2016): 1-11.
- Das, M.P., Devi, P.V., Yasmine, Y. Assessment of in vitro anti-diabetic activity of Ficus glomerata. Der Pharmacia Lettre, 2016; 8 (3): 267-272.
- Mukesh, R., Namita, P. Medicinal plants with antidiabetic potential-A Review. American-Eurasian J Agric Environ Sci., 2013; 13(1): 81-94.
- Roy, A., Prasad, P. Assessment of Antihyperglycemic Potential of Lyophilized and Oven-Dried Extract of Calocybe indica in Experimentally Streptozotocin-Nicotinamide Induced Diabetic Rats. International Journal of Medical Research & Health Sciences, 2016; 5: 4:82-88.
- Roy A, Prasad P. Properties and uses of an Indigenous Mushroom: Calocybe indica. Asian J. Pharm. Tech. 2014; 4(1): 17-21.
- Selvi, S., Umadevi, P., Suja, S., Murugan, S., Chinnaswamy, P. Comparison of non-enzymatic antioxidant status of fresh and dried form of Pleurotus florida and Calocybe indica. Pakistan Journal of Nutrition, 2007; 6(5): 468-471.
CrossRef - Nair, S.S., Kavrekar, V., Mishra, A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. European Journal of Experimental Biology, 2013; 3(1): 128-32.
- Shinde, J., Taldone, T., Barletta, M., Kunaparaju, N., Hu, B., Kumar, S., Placido, J., Zito, S.W. α-Glucosidase inhibitory activity of Syzygium cumini (Linn.) Skeels seed kernel in vitro and in Goto–Kakizaki (GK) rats. Carbohydrate Research, 2008; 343(7): 1278-81.
CrossRef
- Konrad, B., Anna, D., Marek, S., Marta, P., Aleksandra, Z., Józefa, C. The evaluation of dipeptidyl peptidase (DPP)-IV, α-Glucosidase and angiotensin converting enzyme (ACE) inhibitor activities of whey proteins hydrolyzed with serine protease isolated from asian pumpkin (Cucurbita ficifolia). International journal of peptide research and therapeutics, 2014; 20(4): 483-91.
CrossRef - Abirami, G., Nagarani, P., Siddhuraju. In vitroantioxidant, anti-diabetic, cholinesterase and tyrosinase inhibitory potential of fresh juice from Citrus hystrix and maxima fruits. Food Science and Human Wellness, 2014; 3(1): 16–25.
CrossRef - Kumar, S., Narwal, S., Kumar, V., Prakash, O. α-glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacognosy Reviews, 2011; 5(9): 19–29.
CrossRef - Kumar, S., Kumar, V., Prakash, O. Enzymes Inhibition and Antidiabetic Effect of Isolated Constituents fromDillenia indica. BioMed Research International, 2013; 2013: 1-10.
- Lebovitz, H.E. Alpha-glucosidase inhibitors. Endocrinology and Metabolism Clinics of North America, 1197; 26(3): 539–551.
- Kimura, A., Lee, J.H., Lee I.S., et al. Two potent competitive inhibitors discriminatingα-glucosidase family I from family II. Carbohydrate Research, 2004; 339(6): 1035–1040, 2004.
- Picot, C.M.N., Subratty, A.H., Mahomoodally, M.F. Inhibitory potential of five traditionally used native antidiabetic medicinal plants onα-amylase, α-glucosidase, glucose entrapment, and amylolysis kinetics in vitro. Advances in Pharmacological Sciences, 2014; 2014; 1-7.
- Yogisha, S, Raveesha, K.A. Dipeptidyl Peptidase IV inhibitory activity of Mangifera indica. J Nat Prod, 2010; 3: 76-79.

This work is licensed under a Creative Commons Attribution 4.0 International License.





